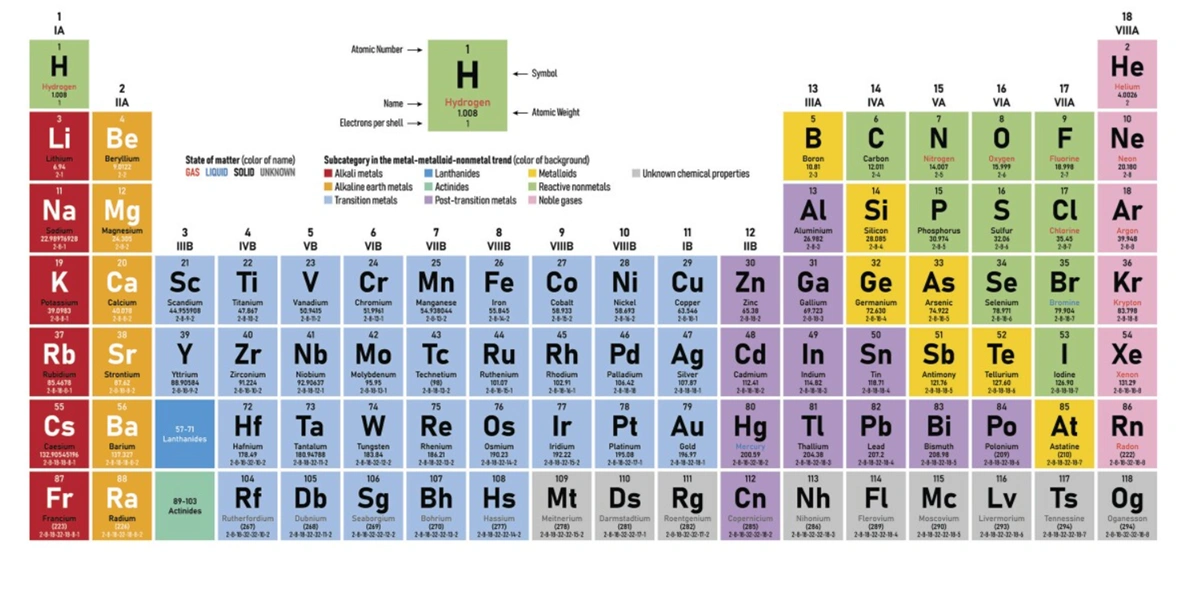
When you think of the periodic table, you probably picture a grid of symbols and numbers representing all the elements known to science. This table is more than just a collection of symbols; it’s a powerful tool that helps scientists understand the properties and relationships of elements. But have you ever wondered which element comes first alphabetically? It’s an intriguing question, especially when we focus on gases. In this article, we’ll dive into the alphabetically first gas on the periodic table and explore everything you need to know about it.
What is alphabetically the first gas on the periodic table?
[sc_fs_faq html=”true” headline=”h2″ img=”” question=”What is Periodic Table?” img_alt=”” css_class=””] The periodic table is a systematic arrangement of elements, organized based on their atomic number, electron configuration, and recurring chemical properties. Developed by Dmitri Mendeleev in 1869, it has become an essential resource in the field of chemistry. Each element has a unique position on the table, providing valuable information about its characteristics. [/sc_fs_faq]
While the periodic table is usually organized by atomic number, sometimes it’s helpful to consider elements in alphabetical order, especially when learning or conducting research. Alphabetical order simplifies finding elements quickly, though it doesn’t reflect the elements’ chemical properties or atomic structure. In scientific contexts, this approach is more of a mnemonic aid than a method for understanding chemical behavior.
The Alphabetically First Gas on the Periodic Table
The element that claims the title of the alphabetically first gas on the periodic table is Argon. Argon, symbolized by Ar, is a noble gas located in group 18. It’s a colorless, odorless, and tasteless gas that makes up about 0.93% of the Earth’s atmosphere, making it the third most abundant gas in our air.
Chemical Properties of Argon
Argon has an atomic number of 18 and an electron configuration of [Ne] 3s² 3p⁶. This configuration makes Argon chemically inert, meaning it doesn’t easily form compounds with other elements. Its lack of reactivity is due to its full outer electron shell, which makes it stable and unlikely to participate in chemical reactions under standard conditions.
Physical Properties of Argon
At room temperature, Argon exists as a gas. It is colorless and odorless, with a density of about 1.78 grams per liter at standard conditions. Argon has a boiling point of -185.8°C and a melting point of -189.3°C. These properties, combined with its inertness, make Argon particularly useful in various applications where non-reactivity is crucial.
Where is Argon Found?
Argon is primarily found in the Earth’s atmosphere, where it is a byproduct of the decay of potassium-40, a radioactive isotope. It can also be found in small amounts in the Earth’s crust and in volcanic gases. Argon is typically extracted from the atmosphere through a process called fractional distillation, which separates it from other gases like oxygen and nitrogen.
Uses of Argon
Argon’s inertness makes it extremely valuable in industrial applications. It’s widely used in welding, where it acts as a shield gas to protect the weld area from atmospheric gases like oxygen and nitrogen, which could cause defects in the weld. Argon is also used in lighting, particularly in gas discharge lamps like neon lights, where it helps to produce a blue or purple glow. Additionally, Argon is crucial in the production of semiconductor materials, where its non-reactive nature ensures a contaminant-free environment.
The Role of Argon in the Atmosphere : In the atmosphere, Argon plays a passive role due to its inertness. However, its presence is important in maintaining the balance of gases. Argon does not react with other elements or compounds, which means it does not contribute to chemical processes that could alter the composition of the atmosphere. This stability helps to buffer against the reactive tendencies of other atmospheric gases.
Health and Safety Aspects of Argon
Argon is generally considered safe and non-toxic. However, like other gases, it can pose an asphyxiation hazard in confined spaces if it displaces oxygen. Therefore, industries that use Argon must implement proper ventilation and safety protocols to prevent accidental asphyxiation. Despite its inertness, handling Argon under extreme conditions, such as in liquid form, requires caution due to the extremely low temperatures involved.
Argon in Modern Technology
Argon’s role in modern technology cannot be understated. It’s used in the manufacture of silicon wafers for electronics, where its inert nature prevents contamination during the production process. In the medical field, Argon lasers are used in surgeries, particularly in eye and skin treatments, due to their precision and effectiveness. As technology advances, the demand for Argon in these and other high-tech applications continues to grow.
The Discovery of Argon
Argon was discovered in 1894 by Lord Rayleigh and Sir William Ramsay. Their discovery was significant because it was the first noble gas to be identified, leading to the later discovery of other noble gases. The discovery of Argon also helped to confirm the existence of a new group in the periodic table—the noble gases—characterized by their inertness and unique properties.
Interesting Facts About Argon
- Argon gets its name from the Greek word “argos,” meaning “inactive,” reflecting its chemical inertness.
- Argon is the third most abundant gas in the Earth’s atmosphere.
- Argon is used in the preservation of historical documents and works of art to prevent oxidation.
Common Misconceptions About Argon
One common misconception is that Argon is just another boring, inactive gas with no significant use. In reality, Argon’s inertness is precisely what makes it so valuable in various industries. Another misconception is that Argon is rare, when in fact, it is quite abundant in the Earth’s atmosphere, although its extraction requires specific techniques.
| Read More Topics |
| Should i take AP bio or AP chemistry for BME |
| Do we know more about organic or inorganic chemistry |
| Theoretical calculation of calorific values |