introduction
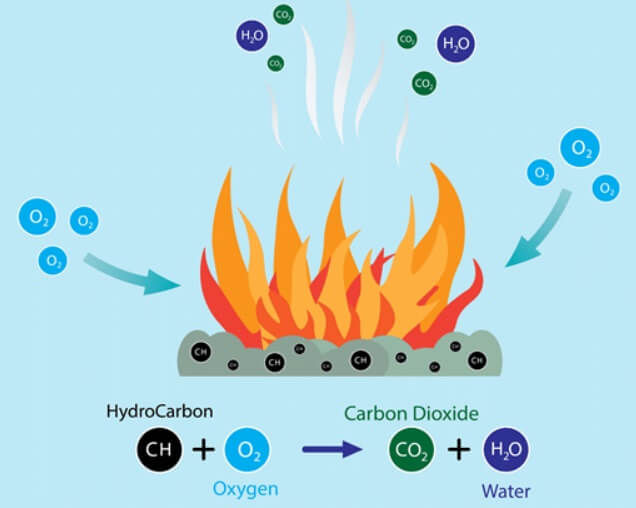
Combustion is a chemical reaction in which a combustible substance burns in air and produce heat and light. This process of heat production is called combustion.
-
Combustion is an exothermic chemical reaction
-
For proper combustion the substance must be brought to its ignition temperature
-
The element generally present in a fuel which undergo combustion are C,H,S and O
-
The oxygen present in air and fuel reacts with C,H and S during combustion of the fuel
-
The minimum quantity of air required for the complete combustion of the fuel can be calculated from the percentage composition of the fuel
Combustion of carbon
Carbon combines with oxygen to give CO2
C2 + O2 → CO2 + 96960 cals 12gms(1) 32gms(8) 44grams
12 gms of carbon needed 32 gms of oxygen, for complete combustion.
Thus the mass proportions of carbon, oxygen and carbon dioxide are 12:32:44 respectively.
12 gms of carbon gives =96960 cals of heat
1gm of carbon would give =96960/12=8080 cals of heat
| Read More Topics |
| Give properties of biodiesel and power alcohol |
| Write note on compressed natural gas |
| Natural gas derived from oil wells of two types |