Based on permanent magnetic dipoles, materials are further classified into
- Antiferromagnetic materials
- Ferrimagnetic materials
Antiferromagnetic materials
Definition: When the magnitudes of all the antiparallel aligned magnetic dipoles of a material are equal and the resultant magnetisation becomes zero, the material is called antiferromagnetic material.
Examples: Manganese oxide (MnO), Chromium oxide (Cr2O3), Ferrous oxide (FeO), etc.
This is first investigated theoretically by Neel and Bitter.
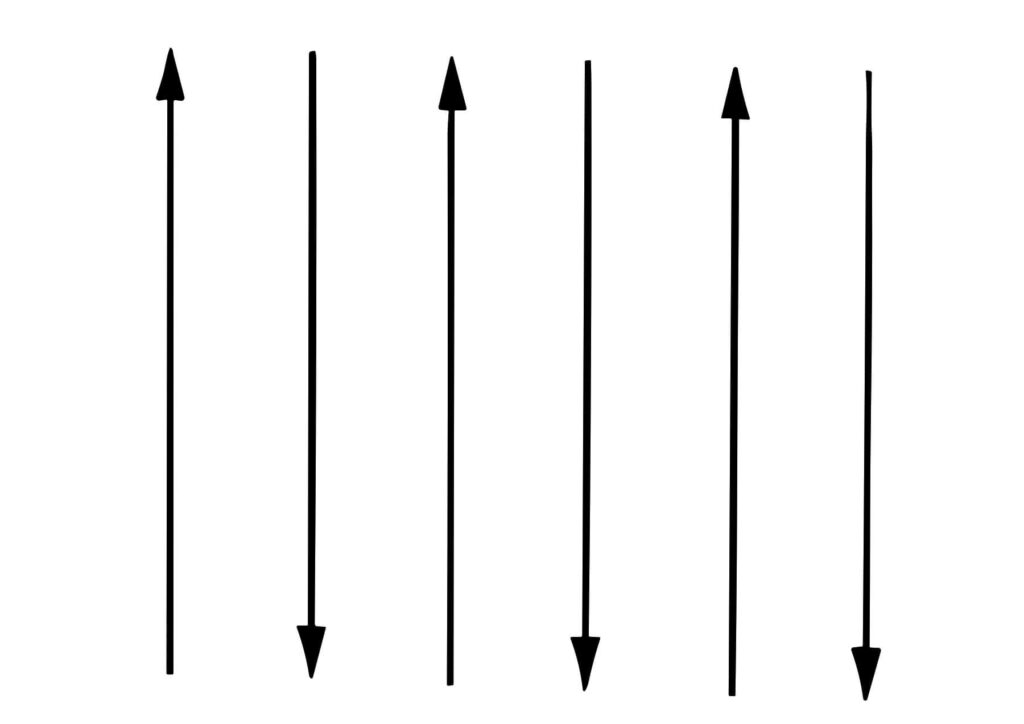
Explanation: In this the spins are aligned in antiparallel manner due to unfavourable exchange interaction among them, resulting in zero magnetic moment. Even when the field is increased, it has almost zero induced magnetic moment.
Antiferromagnetic materials are crystalline materials which exhibit a small positive susceptibility of the order of 10-3 to 10-5.
This variation of susceptibility with temperature follows a peculiar pattern in these materials.
The susceptibility increases with increasing temperature and reaches a maximum at a certain temperature called Neel temperature, TN (Fig (a)).
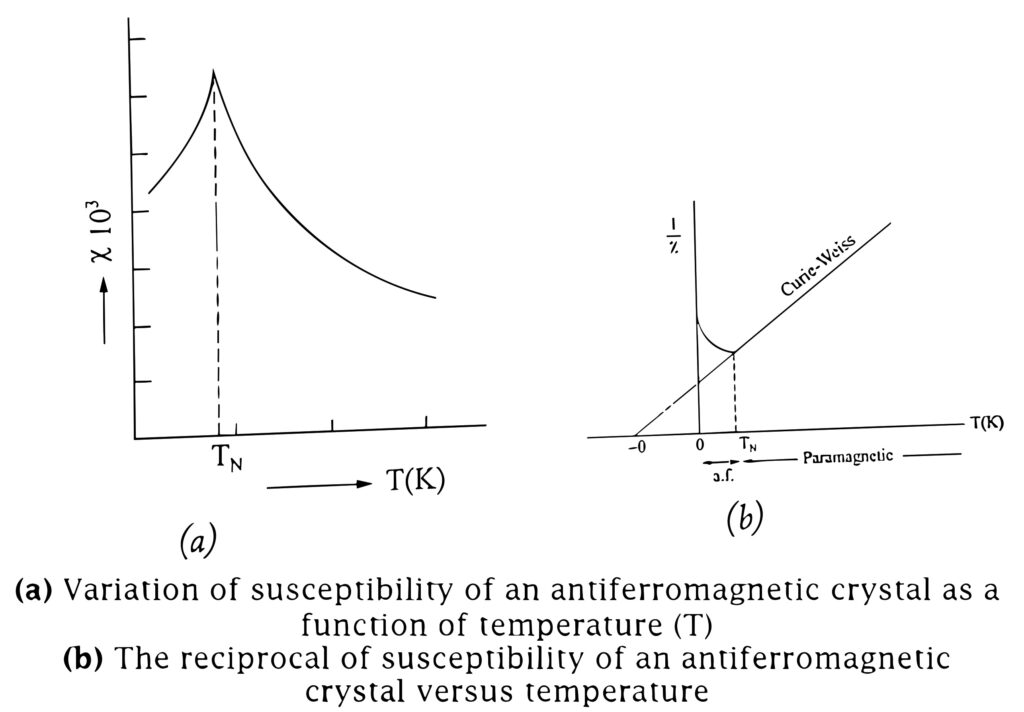
With further increase in temperature, the material goes into paramagnetic state. The material is antiferromagnetic below TN. The transition temperature TN lies below the room temperature for most of the materials.
In the paramagnetic state, the variation of inverse susceptibility (1/χ) with temperature is linear, as shown in Fig (b).
The extrapolation of the paramagnetic line in Fig (b) to 1/χ =0 yields a negative θ. The variation of susceptibility with temperature obeys Curie-Weiss Law. Therefore Curie-Weiss Law is modified as
![]()
where θ is called Paramagnetic Curie temperature and C is Curie constant.
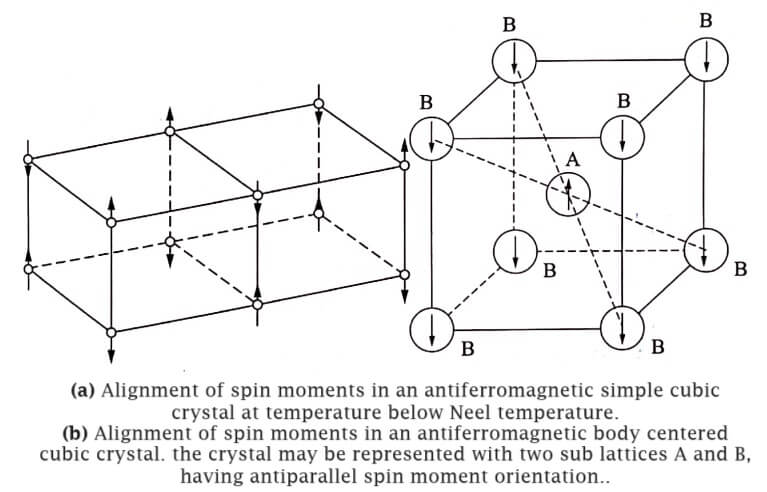
The antiferromagnetic character is explained to be consequence of antiparallel alignment of neighbouring magnetic moments in the crystal as shown in Fig.
As a result, the magnetic moments cancel each others effect.
Properties of antiferromagnetic materials
Electron spin of neighbouring atoms are aligned aligned antiparallel. Therefore net magnetisation is zero.
Antiferromagnetic susceptibility depends greatly on temperature.
With the increase of temperature, the susceptibility of it increase and at Neel temperature susceptibility reaches maximum.
Above the neel temperature, the susceptibility decreases with temperature following the equation
χ = C/T+θ
Where TN – Neel temperature when T<TN
Ferrimagnetic materials (or) Ferrites
Definition: Ferrite is a special type of antiferromagnetic material in which antiparallel aligned dipoles with different magnitudes produce a large net magnetisation.
or
There are substances in which the magnetic moments of the two sub lattices are opposite in direction but not exactly equal in magnitude (Because of the two types of ions in the lattices). Such crystal possess a spontaneous magnetization and exhibit most of the properties of ferromagnets. This is known as Ferrimagnetism.
Materials which exhibit ferrimagnetism are called ferromagnetic materials or ferrites.
Example: Manganese ferrite, nickel ferrite, cadmium ferrite, etc.
Structure of ferrites : Ferrites are the magnetic compounds consisting of two or more different kinds of atoms. Generally ferrites are expressed as X2+, Fe23+, O42- where X2+ stands for suitable divalent metals ion such as Mg2+, Zn2+, Fe2+, Mn2+, Ni2+ etc.
Normally, there are two types of structures present in the ferrites
- Regular spinal
- Inverse spinal
Regular spinal
In the regular spinal type, each metal atom (divalent) is surrounded by four O2- ions in a tetragonal fashion.
For example in Mg2+ Fe23+ O42+ the structure of Mg2+ is given in the fig ang it is called ‘A’ site. Totally in an unit cell, there will be 8 tetrahedral (8A) sites.
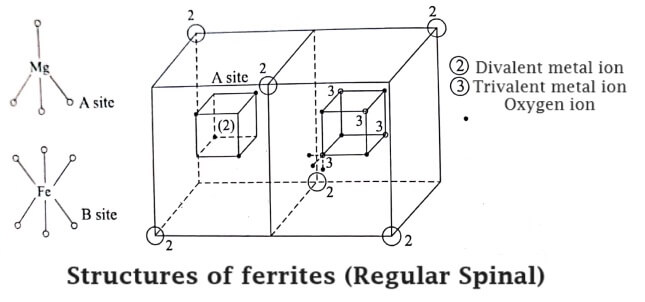
Each Fe3+ (trivalent) is surrounded by ‘6’ O2- ions and forms an octahedral fashion as shown in Fig. Totally there will be 16 such octahedral sites in the unit cell. This is indicated by ‘B‘ site.
Thus, in a regular spinal, each divalent metal ion (Mg2+) exists in a tetrahedral form (A site) and each trivalent metal ion (Fe3+) exists in a octahedral form (B site). Hence, the sites A and B combine together to form a regular spinal ferrite structure as shown in Fig.
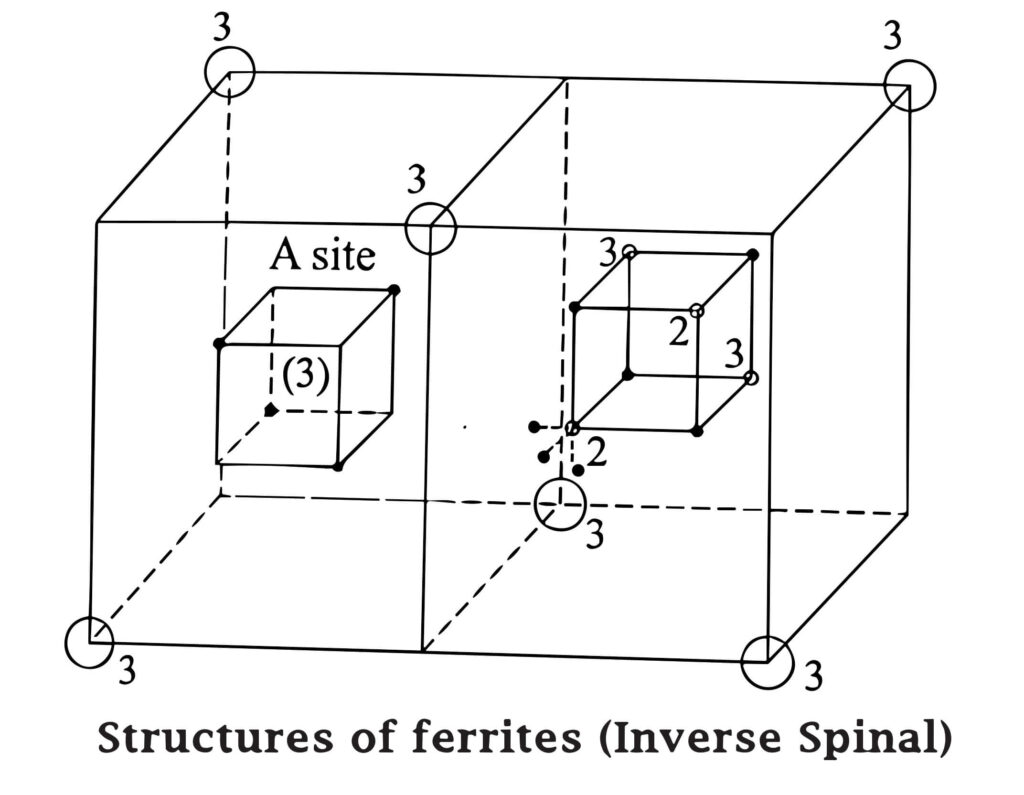
Inverse spinal
In this type, we consider the arrangement of dipoles of a single ferrous ferrite molecule Fe3+ [Fe2+ Fe2+] O42-, Fe3+ ions (trivalent) occupies all A sites (tetrahedral) and half of the B sites (octahedral) also.
Thus, the left out B sites will be occupied by the divalent (Fe2+). The inverse spinel structure is shown in the Fig.
Types of interaction present in the ferrites
The spin arrangement between the A site B site is in an antiparallel manner and it was explained by Neel. According to him, in ferrites, the spin arrangement in antiparallel and there exists some interaction between the A and B sites which is represented as AB interaction.
Apart from this, there are two more interactions (i.e.,) AA and BB interaction which is negative and considerably weaker than AB interaction.
The tendency of AB interaction is to align all A spins parallel to each other and antiparallel to all B spins, but the tendency of AA and BB interaction is to spoil the parallel arrangement of AB spins respectively.
Since AB is very strong as compared with AA and BB, the effect of AB interaction dominates and give rise to antiparallel spin arrangement.
Properties of ferrimagnetic materials
- Ferrimagnetic materials posses net magnetic moment.
- The magnetic dipoles with different spin are aligned antiparallel to each other. So, they have a large magnetisation even for a small external paramagnetic field.
- Above curie temperature, it becomes paramagnetic while it behaves as ferromagnetic material below curie temperature.
- They have a negligible electrical conductivity.
- Mechanically, it has pure ion character.
- They have high permeability and high resistivity.
- They have low eddy current losses and low hysteresis.
Differences between ferrites and ferromagnetic materials
- Ferrites have a very low electrical conductivity as compared o ferrmagnetic materials.
- Ferrites have a higher permeability and lower hysteresis loss as compared to ferromagnets.
Application of ferrites
Manufacture of permanent magnet : Hard magnetic ferrites are used in the manufacturing of permanent magnets which possess high electrical resistance. Such magnets find use in certain applications involving super high frequency technology.
Production of cores : Soft magnetic ferrites are used in the production of cores for inductor coils used in telecommunication and low-power transformers.
Magnetic films : They are used in magnetic films in which demagnetization process occurs at the speed exceeding million times/second. This technology is important for electronics, automobiles and computer hardware engineering.
Magnetic discs and tapes : They are used in magnetic discs or tapes in which the concept of magnetic bubbles is used. The concept of magnetic bubble plays an important role in information storage devices such as magnetic discs and tapes. Magnetic bubbles serve as memory elements in such devices.
They are used to produce ultrasonic waves by magnetostriction principle.
Ferrite rods are used in radio receiver to increase the sensitivity.
Since the ferrites has low hysteresis loss and eddy current loss, they are used in two port devices such as gyrator, circulator and isolator.
Gyrator : It transmits the power freely in both directions with a phase shift of radians.
Circulator : It provides sequential transmission power between the ports.
Isolator : It is also used to display diferentian attenciation.
Ferrite cubes are used in switching circuits and in matrix storage devices and computers.
Ferrites are not metals, but their resistivities lies in the range of insulator or semiconductor. The power losses due to eddy currents is reduced in this type of materials and hence they are used in microwave frequency applications.
| Read More Topics |
| Difference between soft & hard magnetic materials |
| Domain theory of ferromagnetism |
| Definition and properties for ferromagnetism |





