Morphine is one of the most abundant opioid found in opium. It is regarded as the gold standard or benchmark of analgesic used to relieve intense pain. Morphine is named after the Roman god of sleep Morpheus. It is an alkaloid isolated from the juice of unriped seed capsule of the poppy plant Papaver somniferum. Opium contains numerous alkaloids and they are divided into two main types:
- Phenanthrene type: Morphine, Codeine and Thebaine.
- Benzylisoquinoline type, eg. Papaverine, Noscapine.
The Morphine group act on CNS as depressant and stimulant. Papaverine group has antispasmodic action on the smooth muscles. Though Morphine is a potent analgesic, the side effects like, repiratory depression, addiction and sedation associated with Morphine, initiated the attempts for modification of this structure.
Chemistry of Morphine
The structure of Morphine is composed of five fused rings – pentacyclic structure consisting of a benzene ring (A), two partially unsaturated cyclohexane rings (B and C ), a piperidine ring (D) and a dihydrofuran ring (E). Rings A,B and C are the phenanthrene ring system.
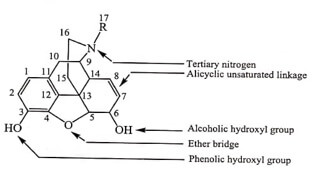
- Two hydroxyl functional groups, a C3 -phenolic OH and a C6 -allylic OH
- An ether linkage between C4 and C5 ,
- Double bond between C7 and C8 ,
- Tertiary amine function at position 17 ,
- It has five chiral centers with absolute stereochemistry 5(R), 6(S), 9(R), 13(S) and 14(R) . The naturally occurring isomer is levo (-) rotatory.
Structure Activity Relationships
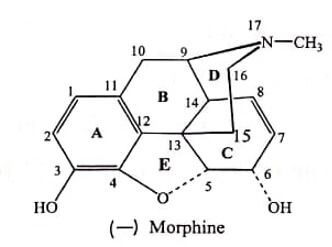
(i) Modification of Ring A
The ring and 3-OH group is crucial for analgesic activity. Any substitution on phenyl ring diminishes activity.
Phenolic OH is essential for binding with μ and κ receptors. Modification of phenolic -OH decreases the analgesic activity.
Altering the C-3 by etherification reduces narcotic analgesic activity and the larger the ether group, the lower the analgesic activity. Codeine (Methyl ether of morphine) is 1/3 less analgesic than Morphine.
Esterification (acetylation) of both the 3- and 6-OH groups yields Heroin, a compound which is both more lipophilic and more potent. But they have greater side effects and have severe tolerance and dependence characteristics.
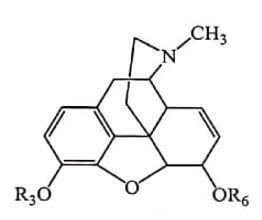
| Compound Name | R3 | R6 |
| Codeine | CH3 – | H- |
| Heroin | CH3CO- | CH3CO- |
(ii) Modification of Ring (alicyclic ring)
The alcoholic hydroxyl group at C-6 when methylated, esterified, oxidized, removed or replaced by halogen, analgesic activity as well as toxicity of the compound increases. In other word 6-OH of Morphine are not required for analgesic activity.
The saturation of the double bond at C-7 position gives more potent compound. eg: Dihydro morphine, Dihydro codeine. This shows that 7,8-double bond of Morphine also is not required for analgesic activity.
Introduction of 14-hydroxy group in dihydro form gives more potent compound. eg 14- Hydroxy dihydro codeinone and 14-Hydroxy dihydromorphinone.
Annelation – Bridging of C6 and C14 through ethylene linkage yields Thebaine compounds such as Etorphine which are 200 times more potent than Morphine potent analgesics. These compounds typically used to immobilize large animals (elephants).
Replacement of the N-methyl group of the Thebaine with a methylcyclopropyl group yields compounds with mixed agonist/antagonist or partial agonist activity. For example, Buprenorphine HC1 (Buprenex) is a mixed agonist /antagonist.
(iii)MODIFICATION OF RING D
Tertiary nitrogen is necessary for good opioid activity.
Replacement of Morphine’s N-methyl group by a hydrogen atom as in Normorphine reduces analgesic activity to 1/8th that of morphine.
Replacement of Morphine’s N-methyl group with larger alkyl groups reduces activity. However, substitution with aralkyl group significantly increases analgesic activity eg N-Phenethylnormorphine.
Replacement of Morphine’s N-methyl group with an allyl group (-CH2-CH=CH2) , a methylcyclopropyl group or a methylcyclobutyl group results in the emergence of opiate receptor antagonist activity.
Three of these compounds – Naloxone, Naltrexone and Nalmefene – are pure opiate antagonists (at μ,k and δ receptors).
(iv)Morphinan Analogues
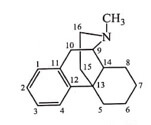
The ethereal linkage is not required for analgesic activity.
Removal of ether (ring E) bridge between the C4 and C5 atom named as Morphinans has increased analgesic activity. The levo form of morphinan possess analgesic activity, while the dextro form is cough suppressant.
Levorphanol and Butorphanol are examples of morphinan series. Levorphanol is eight times more active than Morphine. Butorphanol is a mixed agonist and antagonist.
(v) Benzomorphans or Morphans
Lack of ring C and E of morphine retains the activity and these compounds are named as 6,7- Benzomorphans.
Pentazocine is a classical example from benzomorphan series. It is the mixed agonist-antagonist analgesic to be marketed. It is an agonist at the kappa and sigma opioid receptors and has a weak antagonist action at the mu receptor.
The trimethyl compound ( R1 = R2 : CH3 ) is more active than dimethyl ( R1= H ,R2=CH3 ) compound. N-phenyl ethyl is more active than trimethyl compounds. eg. Phenazocine is approximately 10 times as potent as morphine
(vi) 4-Phenyl piperidine
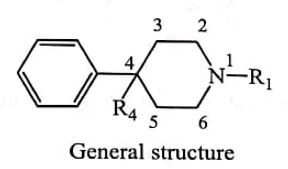
Removal of ring B, C and E (with only A and D) known as 4-Phenyl piperidine class possess analgesic activity, which opened new search of simpler, relatively small and structurally uncomplicated analgesics.
Mepheridine is a major drug from this series with 1/4th of potency of Morphine. The reversed ester of Meperidine, the propionoxy compound was most active, being 5 times more active than Meperidine. eg. Prodines.
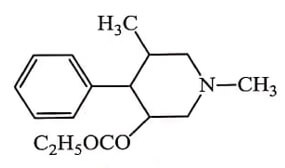
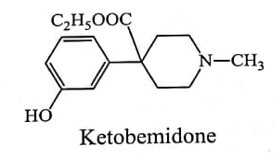
Introduction of m-hydroxy group in phenyl ring increases the activity since it is similar to C3-OH of Morphine eg. Bemidone. Replacement of ester moiety by a ketone function in a Bemidone results in Ketobemidone is equivalent to Morphine in activity.
Structural modifications of 4-Phenyl piperidine lead to the discovery of Fentanyl group which is Morphine like analgesics. Structural modification of Fentanyl, like introducing a small oxygen containing group in 4th position of piperidine ring and replacing phenyl ring with isosteric ring yielded active compounds with lower side effects. Example Alfentanil and Sufentanil.
(vii) Methadone Series
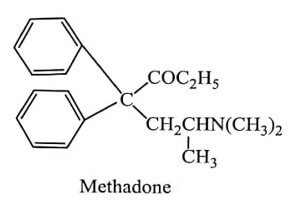
Methadone is orally active and has longer duration of action. The levo isomer of Methadone is twice as effective as its racemates. Many variations in Methadone structure have been made and more useful drugs are analgesic Acetylmethadols and Propoxyphene and antidiarrheal Diphenoxylate.
Replacement of heterocyclic rings like Morpholine and Piperidine for dimethyl amino group gives a compound as potent as Methadone with Morphine like properties. eg. Racemoramide.
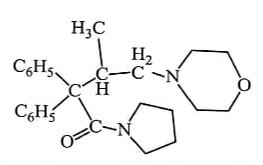
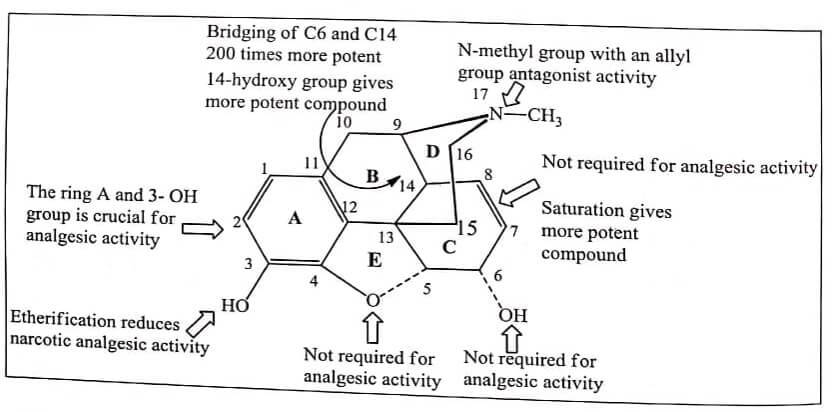 Fig 1.1 Summary of SAR
Fig 1.1 Summary of SAR
Mechanism of action: Morphine causes analgesia by selectively acting on receptor situated both in the higher centre and spinal cord. It activates specific opiate receptors (δ,μ and k ) each of which are involved in controlling different brain functions. Binding of Morphine to the μ opioid receptor results in hyperpolarization of the neuron leading to the inhibition of release of nociceptive neurotransmitters causing an analgesic effect and increased pain tolerance due to reduced neuronal excitability.
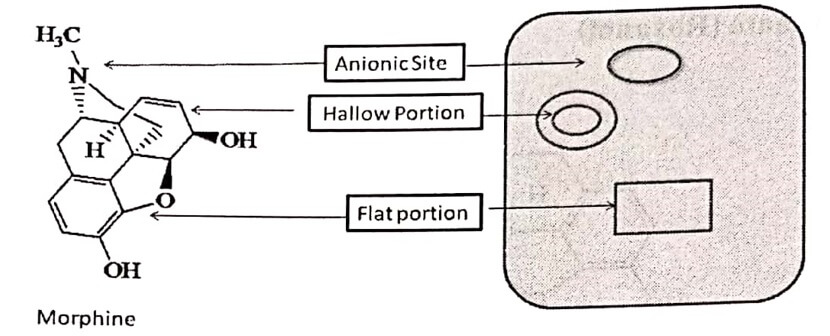
In the central nervous and gastrointestinal systems, Morphine exhibits effects including analgesia, sedation, anxiolysis, euphoria, respiratory depression and gastrointestinal system smooth muscle contraction. The structure features, which are recognized to be essential for the perfect fit of a narcotic analgesic on receptors, are represented below:
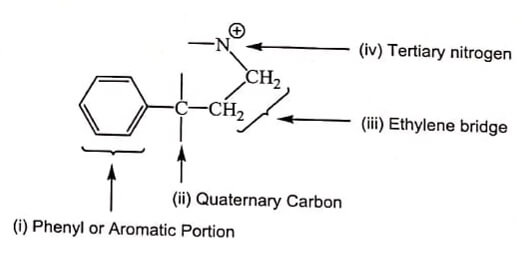 Opioid receptor composed of three prominent parts:
Opioid receptor composed of three prominent parts:- A flat portion which holds aromatic part by Vander-Waal’s force.
- A cavity or a hallow portion which entraps ethylene bridge.
- An anionic site which holds the 3° Nitrogen, which get ionized at physiological pH.
Use: It is used to relieve moderate to severe pain.

Mechanism of action: It is a selective agonist of μ receptor ,but its affinity is weak to opoid receptor. In liver codeine get metabolized to Morphine which is ten times more potent agonist.
Use: It is a mild analgesic and antitussive.
| Read More Topics |
| Application of non-classical bioisosteres in drug design |
| Cholinergic blocking agents or antispasmodics |
| Stereoisomerism and biological activity |





