Electrochemical cells are a cornerstone of modern chemistry and physics. They are the devices that convert chemical energy into electrical energy, playing a crucial role in everything from batteries to large-scale industrial processes. But what happens when a chemist assembles an electrochemical cell? In this comprehensive guide, we will explore the assembly, components, working mechanism, and applications of electrochemical cells.
[sc_fs_faq html=”true” headline=”h2″ img=”” question=”What is an Electrochemical Cell?” img_alt=”” css_class=””] An electrochemical cell is a device that generates electrical energy from a chemical reaction or uses electrical energy to cause a chemical reaction. There are two main types of electrochemical cells: galvanic cells (which produce electricity) and electrolytic cells (which consume electricity). These cells are vital in powering everyday devices and are also utilized in various industrial processes. [/sc_fs_faq]
Importance of Electrochemical Cells in Modern Science
Electrochemical cells have revolutionized modern science by providing efficient energy storage solutions, supporting sustainable energy development, and enabling advanced industrial applications. From powering your smartphone to aiding in the purification of metals, these cells are integral to technological progress.
Types of Electrochemical Cells
Galvanic (Voltaic) Cells
Galvanic cells, also known as voltaic cells, are electrochemical cells that generate electrical energy from spontaneous chemical reactions. Common examples include the batteries used in everyday devices. These cells consist of two different metals connected by a salt bridge and submerged in an electrolyte solution.
Electrolytic Cells
Unlike galvanic cells, electrolytic cells require electrical energy to drive non-spontaneous chemical reactions. These cells are commonly used in electroplating, metal refining, and other industrial processes where a chemical reaction needs to be forced to occur.
Components of an Electrochemical Cell
Electrodes: Anode and Cathode
Every electrochemical cell has two electrodes: the anode and the cathode. The anode is the electrode where oxidation occurs (loss of electrons), while the cathode is the electrode where reduction occurs (gain of electrons). The materials used for these electrodes vary depending on the type of cell and its intended use.
Electrolyte Solutions
Electrolyte solutions are liquids that contain ions and facilitate the flow of electric current by allowing ions to move between the electrodes. In a zinc-copper cell, for example, zinc sulfate and copper sulfate serve as the electrolytes.
Salt Bridge and Its Role
A salt bridge is a tube filled with a salt solution that connects the two half-cells in a galvanic cell. It completes the circuit, allowing ions to flow between the two solutions while preventing them from mixing directly, which would neutralize the cell’s function.
How Electrochemical Cells Work
Oxidation-Reduction Reactions
The heart of any electrochemical cell is its oxidation-reduction (redox) reactions. At the anode, oxidation occurs, releasing electrons, while at the cathode, reduction occurs, gaining electrons. These reactions are what ultimately generate electrical energy in galvanic cells or consume electrical energy in electrolytic cells.
Flow of Electrons and Ions
In an electrochemical cell, electrons flow from the anode to the cathode through an external circuit, providing electrical energy. Simultaneously, ions in the electrolyte solution move to balance the charge generated by this electron flow, ensuring the cell continues to function.
Assembling an Electrochemical Cell
Step-by-Step Assembly Process
- Prepare the Electrodes: Clean the electrodes thoroughly to ensure optimal conductivity.
- Set Up the Electrolyte Solutions: Pour the appropriate electrolyte solutions into two separate beakers.
- Insert the Electrodes: Place the anode in one beaker and the cathode in the other.
- Connect the Salt Bridge: Use a salt bridge to connect the two electrolyte solutions, ensuring ion flow.
- Complete the Circuit: Connect the electrodes to a voltmeter or other measuring device to complete the circuit and begin measuring voltage.
Common Mistakes to Avoid
- Incorrect Electrode Placement: Placing the wrong electrode in the wrong solution can disrupt the chemical reactions.
- Improper Cleaning of Electrodes: Dirty electrodes can increase resistance and decrease the efficiency of the cell.
- Faulty Salt Bridge Connection: Ensure the salt bridge is properly set to avoid direct mixing of electrolytes, which can short-circuit the cell.
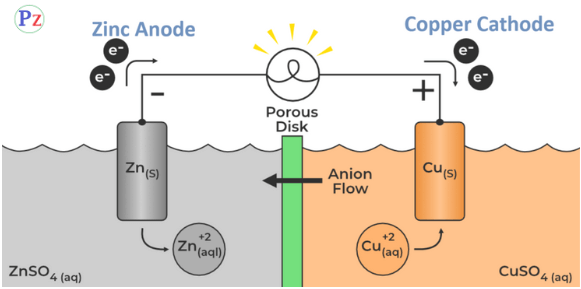
Example: A Zinc-Copper Electrochemical Cell
Materials Needed
- Zinc electrode
- Copper electrode
- Zinc sulfate solution
- Copper sulfate solution
- Salt bridge (usually a potassium nitrate solution)
- Two beakers
- Voltmeter and connecting wires
Procedure to Assemble the Zinc-Copper Cell
- Prepare the Electrodes: Clean the zinc and copper electrodes to remove any surface oxides.
- Set Up Electrolyte Solutions: Fill one beaker with zinc sulfate solution and the other with copper sulfate solution.
- Insert Electrodes: Place the zinc electrode in the zinc sulfate solution and the copper electrode in the copper sulfate solution.
- Connect the Salt Bridge: Place a salt bridge between the two beakers to enable ion transfer.
- Connect the Circuit: Attach wires from each electrode to a voltmeter. The voltmeter should display a voltage, indicating the cell is generating electrical energy.
Applications of Electrochemical Cells
Batteries and Energy Storage
Electrochemical cells are the backbone of battery technology. From household AA batteries to advanced lithium-ion batteries in electric vehicles, they provide portable energy storage solutions essential for modern life.
Electroplating and Metal Refining
Electrolytic cells are widely used in electroplating, where a thin layer of metal is deposited onto a surface, and in refining metals like copper and aluminum, where impurities are removed via electrolysis.
Industrial Applications
Beyond energy storage and metal refining, electrochemical cells are used in industrial processes such as water purification, chemical synthesis, and corrosion prevention.
Factors Affecting the Efficiency of Electrochemical Cells
Temperature and Concentration
The efficiency of an electrochemical cell can be significantly affected by the temperature and concentration of the electrolyte solutions. Higher temperatures generally increase the rate of chemical reactions, while the concentration gradient drives the movement of ions.
Types of Electrolytes
Different electrolytes can impact the conductivity and overall efficiency of an electrochemical cell. Choosing the right electrolyte is essential for optimizing the cell’s performance.
| Read More Topics |
| Alphabetically first gas on the periodic table |
| Theoretical calculation of calorific values |
| Nano electrokinetic assisted paper electrochemical assay |





