Alkaline battery is an improved from of the dry cell. It is introduced in 1949. In this cell, NH4Cl is replaced by KOH as the electrolyte. As the electrolyte in this cell is an alkali, it is called alkaline battery.
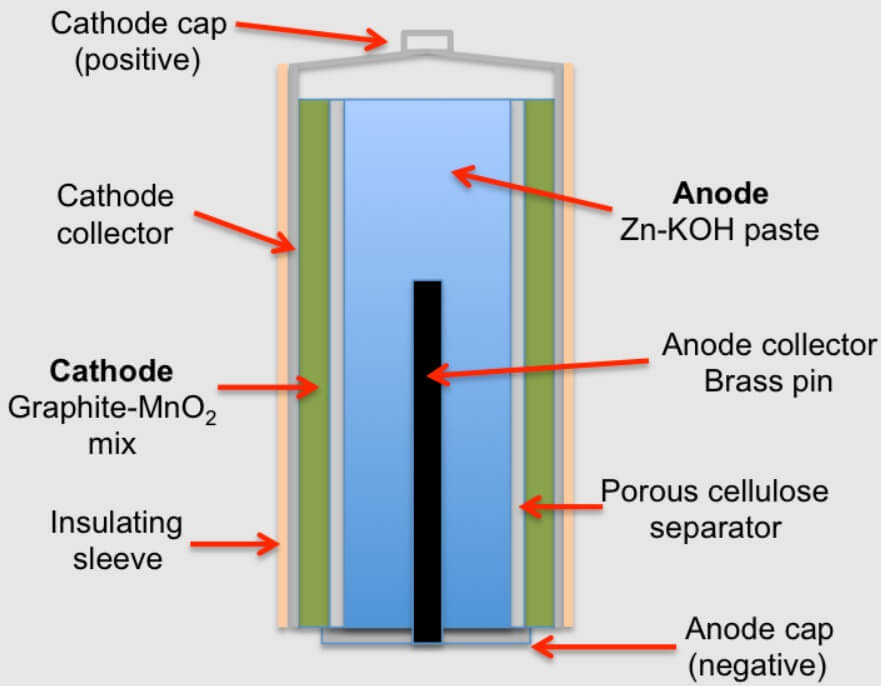
Anode : Zinc
Cathode : Carbon/MnO2
Electrolyte : KOH
In this cell, the cathode is made from a mixture of manganese dioxide and carbon. The anode mix consists of alkaline electrolyte, zinc powder and starch. The alkaline cell derives power from the oxidation of zinc (anode) and the reduction of Mno2 (cathode). The cell reactions are:
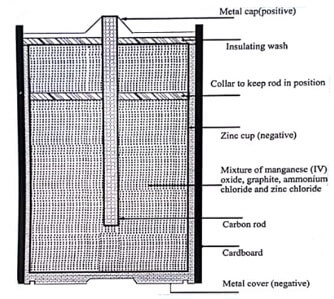
Fig (1) Alkaline battery
| Anode | Zn(s) + 2OH —(aq) → Zn (OH)2(S) + 2e– |
| Cathode | 2MnO2(s) +H2O(1) +2e– → Mn2O3(s)+ 2OH—(aq) |
| Over all reaction: | Zn(s) + 2 MnO2(s) + H2O(1) → Zn(OH)2(s) + Mn2O3(s) |
The alkaline cell gives a voltage of 1 volt to 1.5 volt.
Advantages of alkaline battery
- Zinc does not dissolve readily in a basic medium.
- Its output capacity is high.
- The life of alkaline battery is longer, since there is no corrosion of Zn.
Uses :
(i) It is used in calculators, watches etc.
(ii) It is used in camera exposure control.
Lithium battery
Lithium battery is a solid state battery because solid electrolyte is used in this battery not a solution or a paste.
In this battery the anode is lithium and the cathode is made of TiS2 . The solid electrolyte used is polymer, which permits the passage of ions but not that of electrons.
TiS2 is reduced by lithium. The formation of TiS2 signifies that the Li + ions enters the TiS2 lattice.
The various cell reactions taking place in the Li/TiS2 battery are:
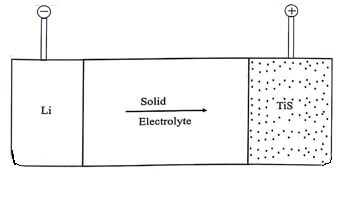
This cell produces a cell voltage of . It is also a type of rechargeable battery.
Recharging the battery
The lithium battery is recharged by supplying an external current which drives the lithium ions back to the anode. The overall reaction is :
LiTiS2 → Li + TiS2
Other secondary lithium batteries
- Li/MnO2
- Li/V2O5
- Li/MoO2
- Li/Cr3O8
Advantages and disadvantages of lithium battery
| Advantages | Disadvantages |
| 1. Lithium cell have high voltages upto about 3V. | 1. Li battery is more expensive than other batteries. |
| 2. Lithium metal is light weight and has good conductivity. | 2. It is very delicate. |
| 3. There is no risk of leakage from the battery, since all the constituents of the battery are solids. | – |
| 4. The energy output of a lithium cell is 2-4 times better than other primary batteries. | – |
Uses
It is used in safety devices, cameras, laptops, computers and many other consumer electronic devices.
| Read More Topics |
| Nuclear reactor definition and components |
| Types of electrochemical corrosion |
| Classification of batteries |