- A classic example of ring versus non-cyclic structure is Diethyl stilboestrol and 17β-oestradiol.
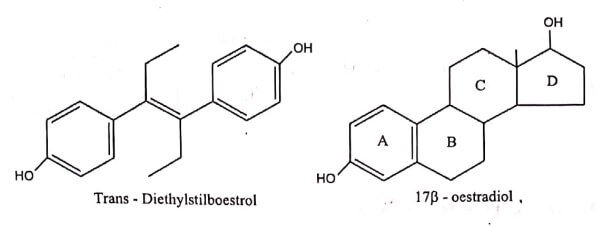
The molecular design of Diethylstilboestrol was carried out by opening the B and C of steroidal nucleus of oestradiol. Due to the presence of double bond Diethyl stilboesterol exist in two fixed stereo isomeric forms; trans-Diethyl stilboesterol estrogenic and cis Diethyl stilboestrol (7% as active).Trans isomer has about the same potency as that of naturally occurring Oestradiol. The central double bond of diethylstilboestrol is highly important for the correct orientation of the phenolic and ethyl groups (trans) at the receptor site.
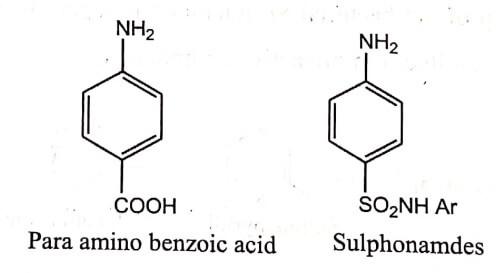
- Antibacterial Sulphonamide derivatives mimic the metabolite Para amino benzoic acid was found during elucidation of its mechanism of action. This similarity was based on electronic and conformational aspects as well as the physicochemical properties such as pKa and logP.
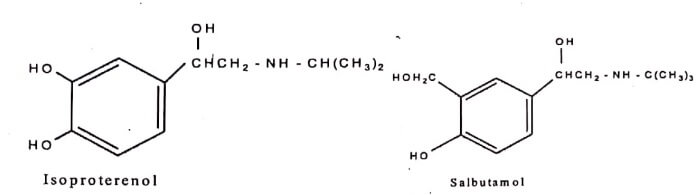
- The 3-hydroxyl group of β-adrenoceptor agonists Isoproterenol, has been replaced with bioisosteric groups −CH2OH,−NHSO2 CH3 and −NHCONH2 gives Salbutamol, Soterenol and Carbuterol respectively. This results in agents with potent and selective activities.
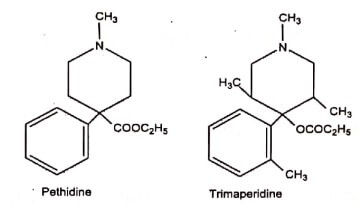
- Trimaperidine is the propanoate ester of a piperidyl alcohol, while Pethidine, Meperidine, is an ethyl ester of a piperidyl carboxylic acid a reversal of an ester group.
-
Isosteric modification of the imidazole nucleus in Cimetidine is Ranitidine without side effects associated with Cimetidine.

Bioisosterism represents one approach used by the Medicinal Chemists for the rational modification of lead compounds into safer and more clinically effective agents. The design of bioisosteres introduces structural changes that can be beneficial or toxic depending upon the size, shape, electronic distribution, polarizability, dipole, polarity, lipophilicity, pKa etc., The development and application of bioisosteres have been adopted as a fundamental approach for number of aspects associated with the design and development. The following properties can be modulated with appropriate bioisosteres
- Greater selectivity
- Improve Potency
- Reducing or redirecting.metabolism
- Less side effects
- Decreased toxicity
- Increased stability
- Simplified synthesis
- Patented lead compounds
Thus bioisosterism has numerous advantageous applications in resolving biological problems effectively.
| Read More Topics |
| Factors affecting protein drug binding |
| Enzymes involved in drug metabolism |
| Clinical significance of protein binding of drugs |





