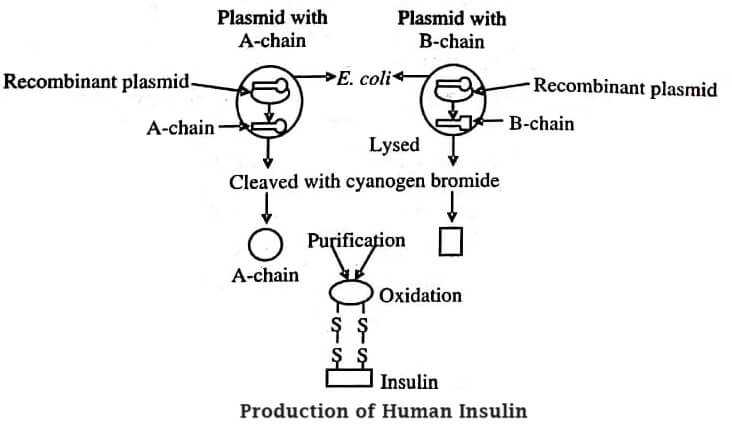
Human insulin was the first recombinant-derived product. It is used for the treatment of diabetes. Fredrick Sanger and his co-worker identified the insulin structure stating that it is made up of two polypeptide chains of A and B with a total 51 amino acid units (21 in A-chain and 30 in B-chain) held by disulphide cross bridges.
Insulin was produced by the chemical synthesis of genes, chains A and B. Both the chains were separately cloned and attached to β-galactosidase, resulting in the synthesis of a fusion polypeptide that is relatively stable in E. coli. Two bacterial strains were created, each producing a fused protein with A and B chains. The genes synthesised lacked promoters. To express the cloned genes into functional protein, a gene is cloned into a plasmid vector close to the bacterial promoter.
A synthetic gene lacks ribosomal binding site, therefore, the gene should be inserted downstream from a promoter and ribosomal binding site of the vector. During the production of synthetic genes, a signal sequences are to be cloned with additional 15-30 amino acids at the N-terminus. These sequences have a central core of hydrophobic amino acids lined with polar or hydrophilic residues.
While passing through the membrane, the signal sequence is cleaved off. The synthetic gene does not contain any methionine residue (initiation codon), thus it is constructed by incorporating methionine residue at the junction of fusion peptide. The accurate insulin can be obtained by cleaving the methionine residue with cyanogen bromide, purifying, and then linking the two chains chemically in vitro (figure).
In another approach to obtain insulin, the gene for precursor molecule was constructed synthetically. The proinsulin gene is cloned to produce a β-galactosidase proinsulin hybrid protein. The proinsulin is chemically cleaved fromβ-galactosidase with cyanogen bromide. Then in vitro proteolytic digestion with trypsin cleaves out amino acids (c-chain) in the middle of the molecule to obtain insulin. The quality assurance of the recombinant insulin involves purity and identity tests.
The insulin obtained from bacterial cell should be identical to human insulin. By sequencing the cloned cDNA, the accuracy of the nucleotide sequence can be established. The primary structure of insulin can be determined from the quantitative amino acid data, and its purity can be established by HPLC.
| Read More Topics |
| Techniques used in recombinant DNA technology |
| Expression of cloned gene within the host |
| Hybrid released translation (HRT) method |