To understand the working principle of laser we must study the quantum process that takes place in a material medium when exposed to light radiation.
When an atom is exposed to (light) photons of energy (hv), three distinct processes take place. They are :
(i) Absorption
(ii) Spontaneous emission
(iii) Stimulated emission
(i) Absorption
If an atom in the lower energy state (E1) absorbs photons of incident light hv , it moves to the higher (ie. upper) energy state (E2) . This transition process is known as absorption. This is upward transition.
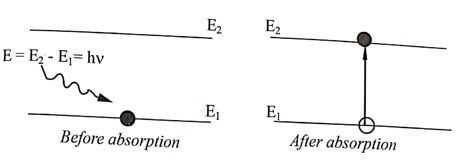
Fig. 1.1 Absorption
The atoms in the excited state will not stay in this state for a longer time. So the atoms in the excited state quickly return to the ground state by emitting a photon of energy hv.
The excited atoms may return to the lower state by
- Spontaneous emission.
- Stimulated emission.
(ii) Spontaneous emission
When an atom in an excited state E2 jumps to lower energy state E1 emitting a photon, without any external influence. Then this transition is called spontaneous emission. This is downward transition.
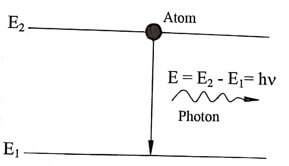 Fig. 1.2 Spontaneous emission
Fig. 1.2 Spontaneous emission
The spontaneous emission is a random process and is an uncontrollable process.
(iii) Stimulated emission
When an atom in an excited state E2 jumps to lower energy state E1 emitting a photon, it emits an additional photon of same frequency of incident photon. This transition is known as stimulated emission. This is downward transition.

Fig. 1.3 Stimulated emission
This concept was first introduced by Einstein.
| Read More Topics |
| Crystal growth techniques |
| Introduction of ultrasonic sound waves |
| Tangent law and tangent galvanometer |





