Cholinergic drugs or Cholinomietics or Parasympathomietics are drugs producing action similar to acetylcholine by interacting with cholinergic receptor or increasing the availability of acetylcholine at these sites.
These agents mimic the action at parasympathetic system. Cholinomimetic drugs are either cholinergic agonist that are resistant to the hydrolytic action of cholinesterase or agents that inhibit cholinesterase.
Structure Activity Relationship
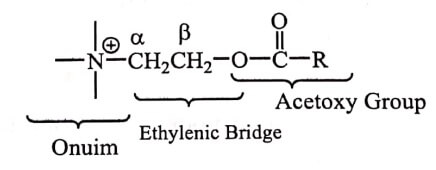
i) Modification of Onium
The trimethyl ammonium group or onium group is essential for intrinsic activity.
Even though trimethyl ammonium group is the optimal functional moiety, some exceptions are known. eg: Pilocarpine, Nicotine and Oxotremorine.
Replacement with 1,2° and 3° amine for trimethyl ammonium group result in less activity.
If one of the methyl group is replaced by ethyl or larger alkyl group the activity decreases.
Replacement of all three methyl group in quaternary amine with ethyl or higher alkyl groups results in antagonist activity.
The replacement of phosphoium, sulfonium or arsenonium isosteres for ammonium ion decreases the activity.
ii) Modification of Acetoxy Group
Ester group is essential for affinity, binds with threonine and asparagine pockets at the receptor site. The ester group of ACh contributes to the binding of the compound to the muscarinic receptor.
When acyl group is substituted by its higher homologues, less active compound is formed.
Since ester group makes it liable to hydrolysis, alternate groups were included and found that replacing the ester with carbamate, ether or ketone function resists hydrolysis while maintaining activity.
When methyl group is replaced by amine (replacing the ester with carbamate) cholinergic activity increases. Carbachol, has both muscarinic and nicotinic properties.
The concept that the ester i.e. carbamic or other group is not essential for activity but may enhances the affinity of the molecule for the receptor.
Studying a series of n-alkyl trimethyl ammonium salt revealed that for maximal muscarinic activity, the quaternary ammonium group should be followed by a chain of five atoms (five – atom rule). (A “rule of five” states that there should be no more than 5 atoms between the Nitrogen and the terminal Hydrogen). As the chain length increased from two, activity is rapidly lost.
iii) Modification of Ethylenic Bridge
Shortening or lengthening the chain of atoms that separates the ester group from the onium moiety reduces muscarinic activity.
Substitution of methyl group on α or β – carbon of ethylenic group result if equipotent or greater cholinergic activity. But larger than methyl group decreases the activity
✓ Introduction of methyl group on the β carbon atom reduces the nicotinic activity but not muscarinic activity (e.g. Methacholine).
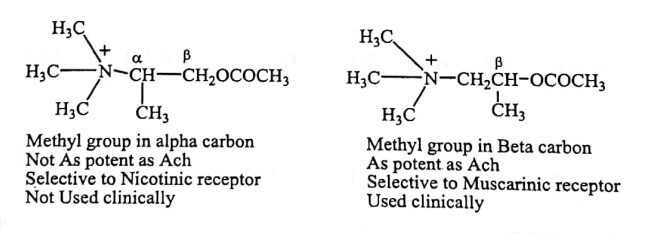
Due to the presence of methyl group on carbon atom, Methacholine (acetyl – β – methyl choline) is longer acting, since it is hydrolysed by acetyl cholinesterase (AChE) enzyme at a much slower rate than acetyl choline and is almost totally resistant to hydrolysis by butyryl cholinesterase (pseudo cholinesterase).
Hydrolysis by acetyl cholinesterase is more affected by β-substitution than α-carbon.
Replacement of acyl group with aromatic or higher molecular weight ester produces cholinergic antagonist effect.
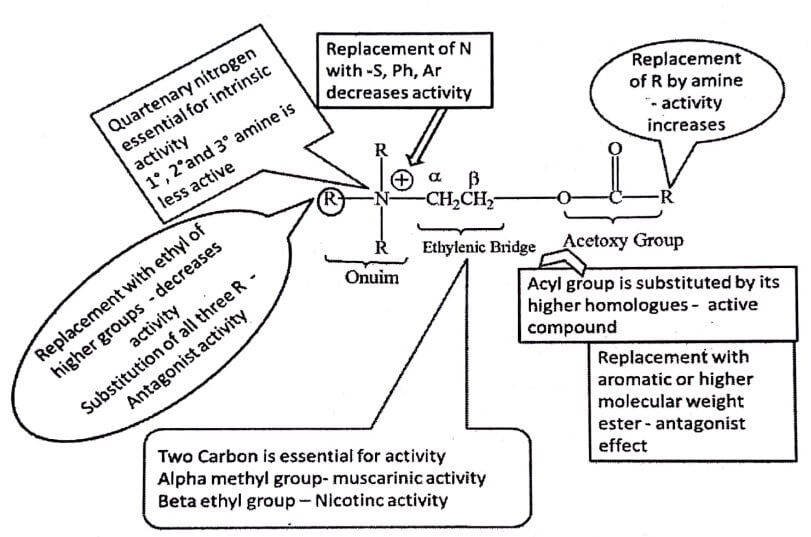
Fig. 1.1 SAR summary of Cholinomimetics
Therapeutic uses: These drugs are used commonly in ophthalmology, where they reduce elevated intraocular pressure in glaucoma and / or induce miosis. In GI disorders that involve, decreased smooth muscle contraction without obstruction. They may be used in the treatment of atonic constipation, congenital megacolon, postoperative intestinal ileus and post- vagotomy gastric atony. Vasospastic peripheral vascular disorders such as Raynaud’s disease and dermatitis congelationis, Sjogren’s syndrome and Alzheimer’s disease also been treated with these agents.
| Read More Topics |
| Application of classical bioisosteres in drug design |
| Stereoisomerism and biological activity |
| Drug dispersio in carriers |





