Batteries are classified into three categories depending upon their recharging capacities:
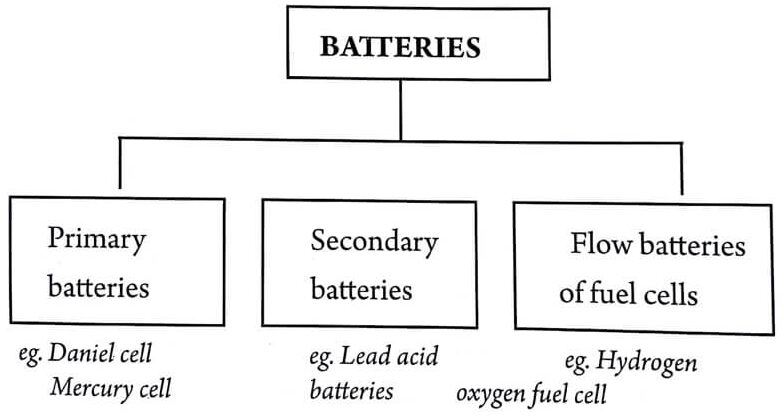
1. Primary battery or primary cells
These are non-rechargeable. Hence, non-reversible. It is meant for a single use and to be discharged after use. Eg: Daniel cell, Leclanche cell, mercury Cell.
2. Secondary battery or secondary cells
These are rechargeable. Hence, reversible. It is meant for a multi cycle use (reusable). Thus a secondary battery may be used through a large number of cycles of charging and discharging. Eg: Lead acid batteries, Ni-Cd battery.
3. Fuel cell or flow battery
The fuel cell is similar to a battery and produce electricity using chemicals. The chemicals used are usually very simple ones such as H2 and O2. Important difference is that fuel cells do not run down like batteries. Eg: H2-O2 fuel cells, methonal fuel cell etc.
Difference between primary and secondary batteries
| Primary Batteries | Secondary Batteries |
| 1. Primary batteries can be used only once and can not recharged. | 1. Primary batteries can be used, recharged and reused. |
| 2. They are cheap. | 2. They are expensive. |
| 3. Initial cost is low. | 3. Initial cost is very high. |
| 4. The chemical reactions that supply the current are irreversible. | 4. The chemical reactions that provide current from the battery are readily reversed when current is supplied to the battery. |
| Read More Topics |
| Solar energy conversion – Energy Sources |
| What is the basic theory of chemical kinetics? |
| Light water reactor nuclear power plant |






