During the last decades the growth of single crystals has assumed enormous importance for both academic research and technology particularly in the field of ‘electronics’. Semiconductor devices made from single crystals are used in the electronics industry for higher performance: In order to use Si for the fabrication of devices, it must be perfect single crystalline form. Single crystals are prepared using crystal growth technique.
“Crystal growth is the process of converting a random oriented or a polycrystalline material into an orderly arranged crystalline material.”
There are number of methods used for the crystal growth technique. This section deals only three crystal growth techniques which are widely used are
- Solution growth
- Melt growth
- Vapour growth
Solution growth Technique
The solution growth method of crystals is the oldest method for growing crystals. It is a low temperature process of growing single crystals. In the solution growth method, a super-saturated solution of the given substance is prepared, and it is kept without any disturbance at a constant low temperature. Single crystals begin to grow from supersaturated solution after some days or week or month. Then the entire supersaturated solution gets converted into a large size single crystals.
The crystallization process is initiated by nucleation. Nucleation is the process in which a very small group of atoms or molecules come together forming sites in the solvent where additional atoms and molecules get deposited and the crystal starts to grow.
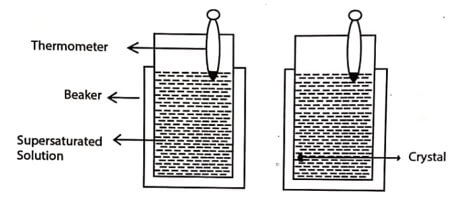
Fig. 1.1 Solution growth technique
The super satturated copper sulphate solution kept in a beaker at room temperature without any disturbance, produces single crystals by the next day.
Advantages:
i. It is a low temperature process.
ii. It is very simple method, not requiring high technology.
iii. This method gives very good quality crystals.
iv. It is also used to avoid the thermal shock during growth of the crystals or on removal from the apparatus.
Disadvantages:
i. Growth rates are very low.
ii. The use of this method is limited only to those materials which are significantly soluble in a solvent and have temperature dependent solubility.
MELT GROWTH TECHNIQUE
Melt growth is the process of crystallization by fusion and resolidification of the pure material by cooling the liquid below its freezing point.
The melt growth method is the most important method of crystal growth. The growth from melt can further be sub-grouped into various techniques. The main techniques are,
(i) Bridgman technique
(ii) Czochralski technique
(i) Bridgman technique
This technique was named after its inventor (Bridgeman 1925). It is one of the method used to prepare single crystals.
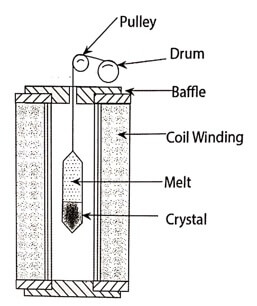
Advantages:
ii. Shape and size of the crystals can be conveniently controlled.
iii. This method is best suited for low melting point materials.
Disadvantages:
ii. This method cannot be used for the materials which decompose before melting.
iii. During cooling process, compression will induce some stresses in the crystal and this will lead to lattice defects.
(ii) Czochralski Technique
Czochralski method is one of the most popular and widely used industrial methods for growing single crystals of semiconductors. It is named after its inventor Czochralski.
Principle:
This method is crystal pulling technique from the melt. The process is based on a liquid – solid phase transition driven by a seed crystal in contact with the melt.
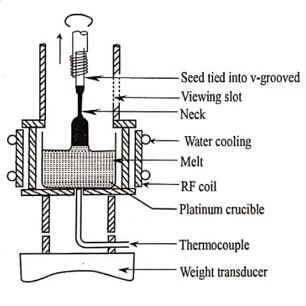
Fig. 1.3 Czochralski technique
Working:
The rate of pulling the seed crystal is determined by the trial and error method. Once the correct value of the rate of pulling of the seed crystal is determined, the single crystals can be readily reproduced. In semiconductor industry, this method is used to produce silicon crystal of size 200mm to 300mm diameter and length more than 1m .
Advantages:
- Thermal units of Czochralski method is simple technique.
- By this method large single crystals can be obtained.
- High crystalline perfections can also be achieved.
- Diameter of the crystals can be varied by varing the thermal parameters with the rate of growth.
Disadvantages:
- This method is not suitable for incongruently melting compounds:
- This method is not easily adaptable to continuous growth.
- Primary difficulties are associated with engineering problems in accommodating rotation and pulling of the crystal.
Vapour growth technique
The growth of single crystal material from the vapour phase is probably the most versatile of all crystal growth processes.

The commercial importance of vapour growth is the production of thin layers by chemical vapour deposition (CVD), where usually irreversible reactions (e.g.) decomposition of silicon halides or of organic compounds are used to deposit materials epitaxially on a substrate. Doping can be achieved by introducing volatile compounds of dopant elements into the reaction region. The thickness of the doped layer can be controlled.
Advantages:
ii. It is used to produce thin layers.
iii. Allotropic crystalline forms can be grown.
iv. Good control of stoichiometry is possible.
Disadvantages:
i. Deposition on substrate as well as on the walls of the container.
ii. Reactive gases are dangerous is some cases and they need special handling procedure.
iii. This method requires high temperature in the order of 800°C to 1250°C.
| Read More Topics |
| Introduction of ultrasonic sound waves |
| Difference between TEM and SEM |
| Tangent law and tangent galvanometer |