This method relies on the diffusion of an antibiotic from a vertical cavity or a cylinder 2 through the solidified agar layer in a petri plate. The growth of test microorganisms is observed to be inhibited in a circular area or zone around the cavity containing antibiotic solution.
The steps involved in cup-plate method are given below:
A liquefied assay medium (43−45°C) is inoculated by the suspension of test microorganisms.
This inoculated test culture medium is poured and spread on sterile petri plates or pre-prepared agar plates.
Standard and test antibiotic solutions of known concentrations are prepared in appropriate solutions, which are then added to sterile cavities prepared on solid medium (figure).
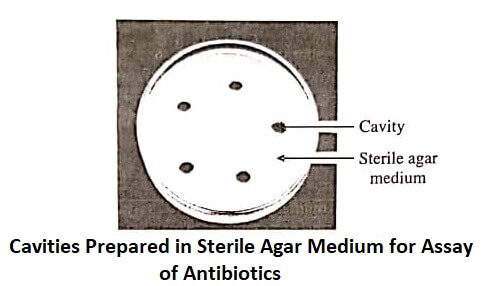
Uniform volume of solution should be added to each cavity to fill them sufficiently. If paper discs are used, they should be sterilised first, then dipped in the standard or test solutions, and finally placed on the medium surface.
The plates are allowed to stand at room temperature or at 4°C for 1−2 hours. This is the period of pre-incubation diffusion which minimises the effects of variation in time between the applications of different solutions.
All plates are then incubated at the temperature listed in table 11.3 for 18-24 hours.
The diameters or areas of the circular inhibition zones (figure) produced by standard and test antibiotic solutions are accurately measured.
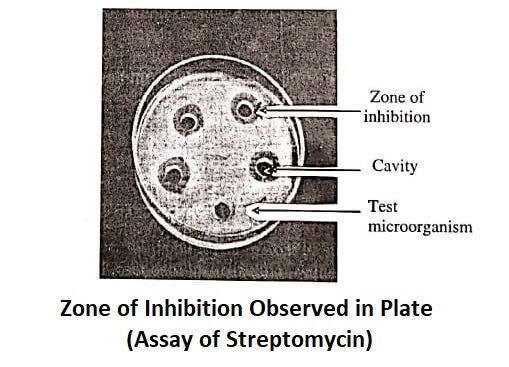
In cup-plate method, the assay designs should be selected based on the requirements stated in individual monographs. Some of the common assay designs are discussed below:
One-Level Assay with Standard Curve: This is carried out as follows:
Standard Solution: It is prepared as follows:
An accurately weighed and previously dried standard antibiotic preparation is dissolved in the specified solvent, and diluted up to the desired concentration to obtain the stock solution.
It is then stored under refrigeration and should be used within the specified period.
The day on which assay has to be performed, 5 dilutions (solutions S2 to S5) representing five test levels of the standard and increasing stepwise in the ratio of 4:5 are prepared from the stock solution.
Sample Solution: The information available for the test antibiotic preparation (the “unknown”) is used for assigning an assumed potency per unit weight or volume. Based on this assumption, a stock solution with the same solvent as used for the standard is prepared on the day of assay. A dilution up to a concentration equal to the median level of standard is prepared from this stock solution to give the sample solution.
Method: The standard curve is prepared using 12 petri plates for 72 cavities (or cylinders). The steps involved are:
A set of 3 plates (having 18 cavities) are used for each dilution.
On each plate, the alternate cavities are filled with solution S3 (representing the median concentration of standard solution) and the remaining 9 cavities are filled with one of the other 4 dilutions of the standard solution.
The process is repeated for the other 3 dilutions of the standard solution.
For each unknown preparation, a set of 3 plates (having 18 cavities) are used, their alternate cavities are filled with sample solution, and the remaining 9 cylinders are filled with solution S3.
The plates are incubated at the specified temperature for 18 hours.
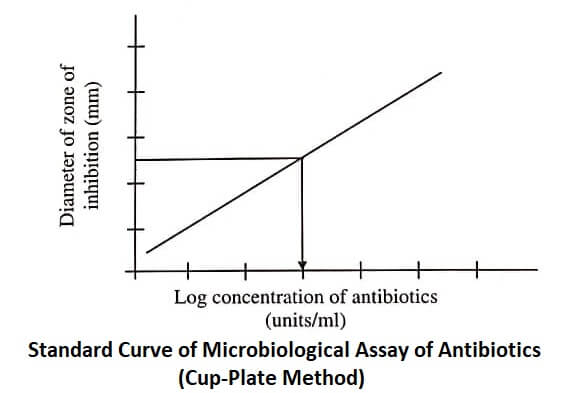
The diameters of the zones of inhibition are measured, plotted against the logarithm of antibiotic concentration (figure), and the concentration of test antibiotic is calculated from the graph obtained.
Two-Level Factorial Assay: Parallel dilutions containing two levels of both the standard (S1 and S2) and the unknown (U1 and U2) solutions are prepared. Four or more plates, each having 4 cavities are alternately filled with standard and unknown solution. The plates are then kept at room temperature and the diameters of the zones of inhibition are measured.
Other Designs: These include:
- Factorial assay having parallel dilution of three test levels of standard and the unknown, and
- Factorial assay involving two test levels of standard and two test levels of two different unknowns.
| Read More Topics |
| Isolation method for pure culture of bacteria |
| Single cell isolation methods |
| Evaluation of efficiency of sterilization methods |
| Morphological classification of bacteria |