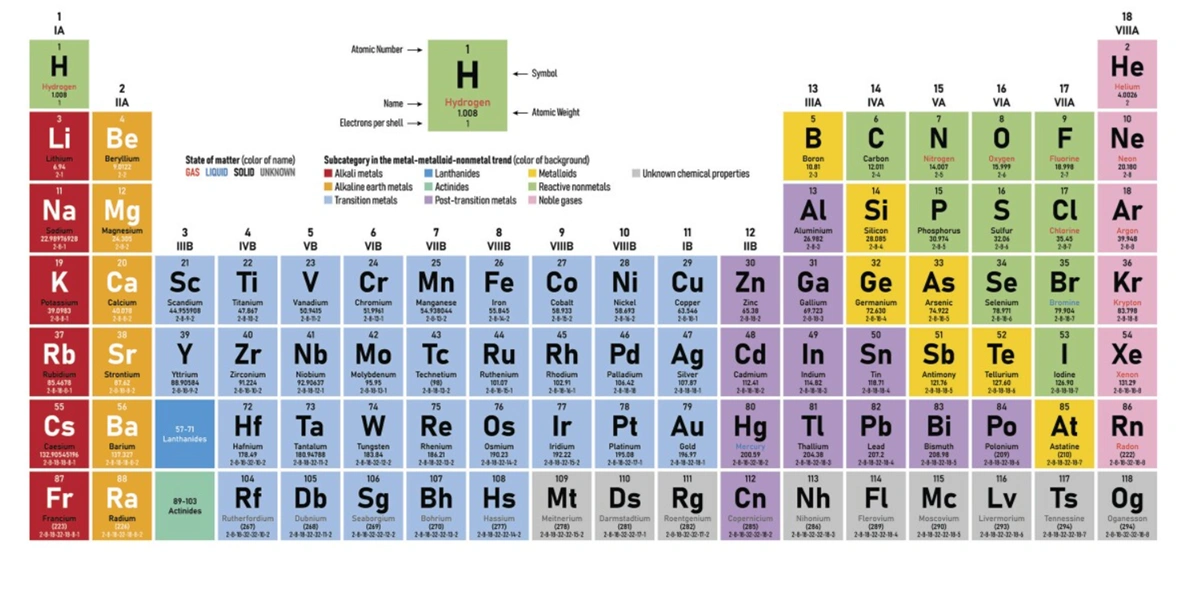
Calorific value of fuels
Calorific values is defined as “the amount of heat liberated in calories by the complete combustion of the fuel with oxygen and the product of combustion to the desired temperature.
Calorific value of fuel is its heating value. Higher the calorific value of fuel greater is its fuel value.
Units of calorific value
There are many units of calorific value. They are
(i) Calorie, (ii) Kilocalorie, (iii) British thermal unit and (iv) Centigrade heat unit (C.H.U)
(i)‘Calorie’ is defined as “the amount of heat required to raise the temperature of one gram of water through one degree centigrade” (15-16°C)
(ii) “Kilocalorie” is defined as “the quantity of heat required to raise the temperature of one kilogram of water through one degree Centigrade”.
Thus 1kCal = 1000 Cal.
(iii) “British Thermal Unit” ( B.Th.U) is defined as “the quantity of heat required to raise the temperature of one pound of water through one degree Fahrenheit” (60°F -61°F ).
1Cal/g = 1KCal/Kg =1.8Btu/1b
1 Btu/1b = 0.55 cal/g
| Read More Topics |
| Give the composition and uses of producer gas |
| Advantages and disadvantages of gaseous fuels |
| Fraction by distillation of crude petroleum |