Extent of antimicrobial action, rate of disinfection, and potency of a disinfectant depends on the following factors:
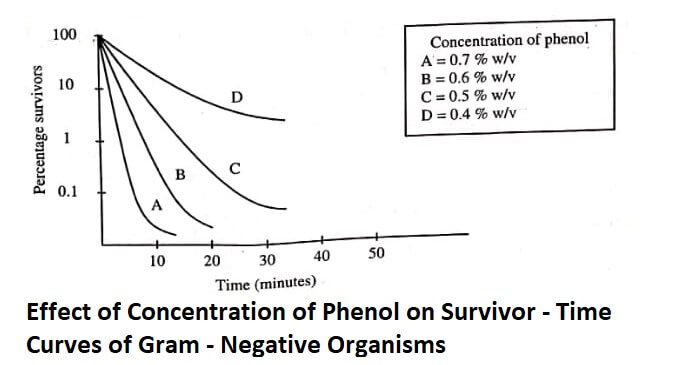
Concentration of Disinfectant : Concentration of the disinfectant directly influences the rate of killing microorganisms. The curve obtained between the efficiency and concentration of disinfectant is generally exponential, rather than linear.
Temperature : Normally, on increasing the temperature the rate of disinfection increases. Temperature coefficient (Q10) is used for quantitative representation of temperature effect on bacterial activity. Thus,
![]()
![]()
Where, T2 and T1= Two temperatures differing by 10°C
t2 and t1 = Corresponding lethal times
Time of Exposure : Each disinfectant takes sufficient time of contact to produce its action and to explain this first-order kinetics. The velocity or rate constant (K) is the measurement of the efficiency of the disinfectant:
![]()
Where, t = Time for the viable count to fall from N0 to N1
N0 = Initial number of microorganisms
Nt = Final number of microorganisms
The survivor/time curve is not constant; and shape of the curve mainly depends on the concentration of the disinfectant (figure):
The pH of Environment : The rate of growth of inoculum, and the potency and ability of the disinfectant to combine with the cell surface during the disinfection process depends on the pH changes.
Surface Tension : The surface properties influence the contact between the aqueous solutions of disinfectants. These surface properties help in adsorption of surface active disinfectants on the surfaces of cell, and also in spreading and wetting properties of the solution.
Formulation of the Disinfectant : Formulation of a disinfectant is an important factor as it influences the effective use of disinfectant. For example, quaternary ammonium compounds and chlorhexidine are efficient in 70% alcohol than in aqueous solution.
Chemical Structure of Disinfectant : Any change in the molecular structure of the chemical compound may alter the activity and efficiency of the disinfectant. For example, para-substitution of an alkyl chain up to 6-carbons in length increases antimicrobial activity, but greater than 6-carbons in length decreases water solubility and disinfectant activity.
Type and Number of Microorganisms Present : Number and nature of the contaminating microorganisms directly influence the efficiency of disinfection. The presence or absence of bacterial spores directly affects the efficiency of disinfectant, as most of the disinfecting agents are ineffective on bacterial spores and viruses.
Interfering Substances in the Environment : Presence of many compounds (e.g., body fluid, food residues, blood, milk, pus, or colloidal proteins), even in small amount may decrease the effectiveness of many disinfecting agents.
Synergism, Antagonism, and Potentiation of Disinfectants : Synergism or synergistic effects are often shown by two antimicrobial agents which gives an increased activity. Antagonism or antagonistic effects result in decreased antimicrobial activity and is used in the elimination of antimicrobial properties of materials (tested for sterility), e.g., sodium thiosulphate, lubrol W+ lecithin, etc. Potentiation of a disinfectant leads to enhanced antimicrobial activity, e.g., polysorbate 80.
| Read More Topics |
| Embryonated hen’s egg introduction |
| Sterilisation of fermentation Air |
| Evaluation of efficiency of sterilization methods |
| Quantitative measurement of bacterial growth |