Flocculated and deflocculated suspension: The solid drug is crushed into smaller particles and then dispersed in a dispersion medium in order to prepare a suspension. As a result of crushing, the surface area of particles and their associated surface free energy increases. This makes the system thermodynamically unstable with highly energetic particles which regroup and reduces the total free surface energy.
Therefore, the particles dispersed in the liquid suspension form light, fluffy floccules held together through weak Van der Waal forces.
In the presence of stronger adherent forces, the particles are known to form aggregates. So, flocculation may result in aggregation.
After the particle size is reduced, the free surface energy (ΔF) of particles increases with increase in their surface area (ΔA). This can be expressed as:
ΔF = γ . ΔA
Where, γ= Interfacial tension between liquid medium and solid particles.
A suspension can attain thermodynamic stability either by:
- Decreasing the interfacial tension, or
- Reducing the total surface area.
The second method is rarely employed to attain thermodynamic stability in pharmaceutical suspensions. On adding a wetting agent, reduction in ΔF value is observed since the agent gets adsorbed at the interface between the particles and the vehicle, and reduces the existing interfacial tension.
In addition to the free surface energy, the particles in a liquid medium also exhibit attractive and repulsive forces. The magnitude of these forces determines if the particles should come in contact with each other or get repelled. The repulsive forces arise in the presence of an electrical potential of the surrounding medium.
The particles acquire a charge due to the following reasons:
- Surface ionisation of the molecules,
- Ions can be adsorbed by the particles in the surrounding liquid, or
- A difference existing between the dielectric constants of the dispersion medium and the dispersed particles.
Zeta potential is known to exist at the particle surface. In case of high zeta potential, the attractive Van der Waal forces surpass the repulsive electrical forces between the two particles.
The deflocculated particles are dispersed in this manner. Besides lowering the zeta potential, addition of an adsorbed ion carrying a charge opposite to that of the particles is also known to neutralise the surface potential.
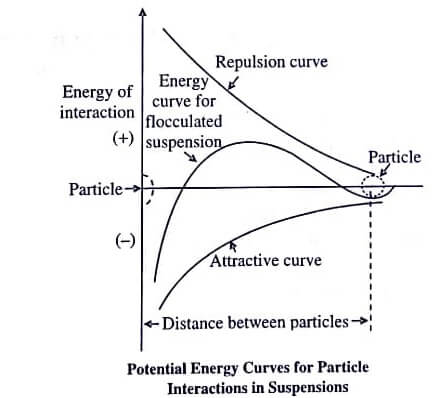
At a certain concentration of the added ions, the attraction forces are known to exceed the electrical forces of repulsion. Hence, the molecules closely interact with each other and tend to form floccules. This sort of system is known as a flocculated system.
However, if the flocculating agent is added continuously in high concentration, the zeta potential may increase in opposite direction and may reverse the above process. Flocculating agents usually comprises of electrolytes, polymers, and surfactants. Figure generally depicts the energy diagram presenting the above mentioned approach.
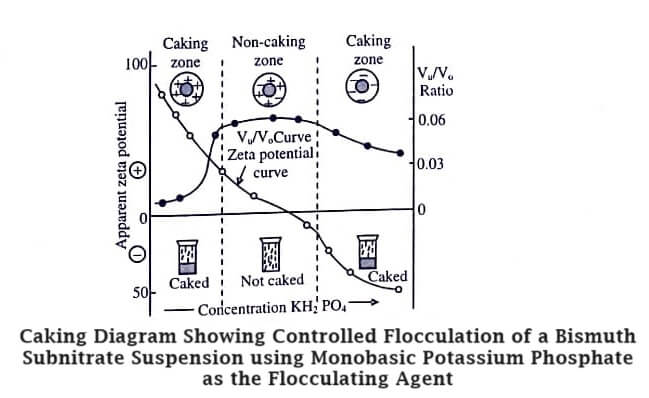
Figure presents the caking diagram depicting the flocculating agent role, e.g., role of monobasic potassium phosphate in the flocculation of a bismuth sub-nitrate suspension.
The table signifies the differentiating points between deflocculated and flocculated suspensions:
Table: Difference between Deflocculated and Flocculated Suspensions
Table: Flocculated and Deflocculated Suspension
| Deflocculated Suspension | Flocculated Suspension |
| Pleasant appearance because of the uniformly dispersed particles. | Unpleasant sediment and clear supernatant layer. |
| Cloudy supernatant is obtained. | A clear supernatant is obtained. |
| Repulsive forces exist between the particles. | Attractive forces exist between the particles. |
| Particles form separate entities. | Particles from loose aggregates. |
| Sedimentation rate is slow as the particle size is small. | Sedimentation rate is high as the flocs are a collection of smaller particles (higher size). |
| Particles settle independently and separately. | Particles settle as flocs. |
| The sediment is closely packed, thus, forms a hard cake. | The sediment is loosely packed, thus, does not forms a hard cake. |
| Re-dispersing the hard cake is not possible. | Re-dispersing the sediment is easy. |
| In the potential energy curves, it represents the primary minimum. | In the potential energy curves, it represents the secondary minimum. |
| Achieves high bioavailability. | Achieves comparatively low bioavailability. |
What is the formulation of flocculated and deflocculated suspension?
The formulation of flocculated suspension includes flocculating agents that help particles form loose aggregates, preventing caking and making redispersion easier. In contrast, a deflocculated suspension uses dispersing agents to keep particles separate, resulting in a uniform mixture but with a risk of hard sediment formation. Choosing the right formulation depends on the desired stability and ease of use in pharmaceuticals and other industries.
| Read More Topics |
| Large scale production fermenter design |
| Enzyme linked immunosorbent assay (ELISA) |
| Large scale production of MAbs |