Manufacture of metallurgical coke
There are two methods of manufacturing metallurgical coke which are named according to the character of the oven in which the distillation is carried out.
Synthesis of coke by Otto-Hoffman’s by product oven method
Construction
The by-product coke oven consists of a number of narrow silica chambers. Each chamber is about 10−12 m long, 3−4 m height and 0.4-0.45m wide. Each chamber is provided with a charging hole at the top. It also acts as an out-let for coal gas which is produced during carbonization. It is also provided with an iron door at each end for discharging coke (Fig. 1).
Working
The Otto oven works on the basis of regenerative system of heat economy i.e., the waste gas produced during carbonisation is utilised for heating the heat regenerators or checker-brick works,
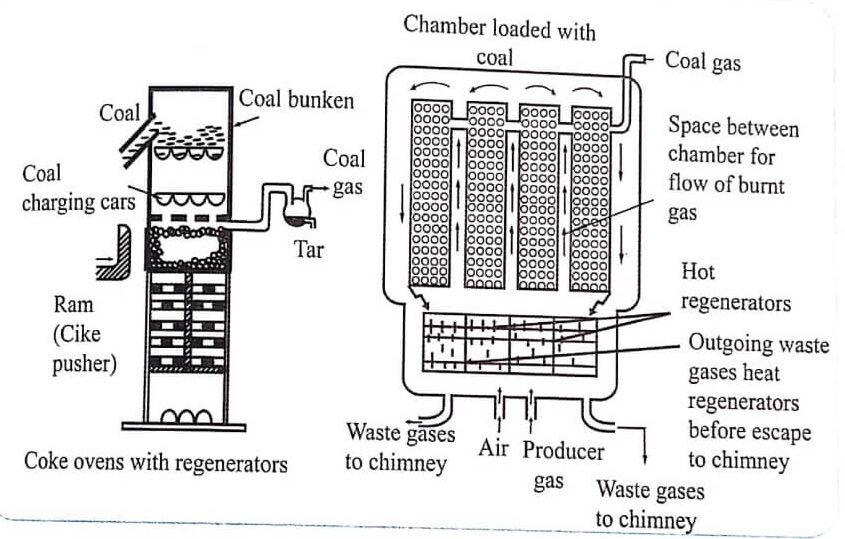
Fig.1 Otto Hoffman’s by-product coke oven with regenerators
Coal is introduced from the top. The ovens are charged from the top and are closed to restrict the entry of air. The chambers are heated to 1200°C by burning the preheated air and the producer gas mixture in the interspace between the chambers.
The air and producer gas are preheated by passing them through second and third hot regenerators. To achieve economical heatings, the direction of inlet gases and waste gases are changed frequently. Carbonisations takes about 12-20 h to complete. When the process is complete, the coke is removed and quenched with water.
The gas coming out from ovens during carbonisation is called coke oven gas. It is mainly composed of coal gas, tar, ammonia, benzol and other aromatic compounds, H2 S, etc.
Recovery of by-products
The gas coming out from the oven is known as “coke oven gas” and is mainly composed of tar, ammonia, naphthalene, benzene, H2S, moisture etc.
(i) Recovery of tar :
The gas is first passed through a tower in which liquor ammonia is sprayed. Here dust and tar get collected in a tank below, which is heated by steam coils to recover back the sprayed ammonia. This ammonia is used again.
(ii) Recovery of ammonia :
The gases from the chamber are then passed through another tower in which water is sprayed. Here ammonia gets converted into ammonium hydroxide solution.
(iii) Recovery of napthalene :
The gases are then passed through another tower in which water at very low temperature is sprayed. Here naphthalene gets condensed.
(iv) Recovery of benzene :
The gases are then sprayed with petroleum, when benzene and its homologues are removed.
(v) Recovery of H2S :
The gases are then passed through a purifier which is packed with moist Fe2 O3. Here H2S is retained.
Fe2O3 + 3H2S → Fe2S3 + 3H2O.
Once all Fe2O3 is changed into Fe2S3, the purifier is exposed to atmosphere, when Fe2O3 is regenerated.
Fe2S3 + 4O2 → 2FeO + 3SO2 ↑
4FeO + O2 → 2Fe2O3
The final gas left out is called as coal gas which is used as a gaseous fuel.
Advantages of Otto-Hoffman’s method
-
Valuable by-products like ammonia and coal gas are recovered.
-
Heating is done externally by producer gas.
-
The carbonisation time is reduced.
| Read More Topics |
| Explain briefly the manufacture of glass |
| What is white cement – Give its properties and uses |
| Composition and properties of glass |





