Fuel cells
A fuel cell is an electrochemical cell, which can convert the chemical energy of the fuel into electrical energy.
It is an energy conversion device or electricity generator.
A fuel cell operates like a galvanic cell. This is capable of supplying current as long as it is provided with the supply of reactants. Fuel cells convert about 75% of the available chemical energy into electrical energy.
Fuel + Oxygen → Oxidation product + Electricity
E.g., Hydrogen-oxygen fuel cell; alkaline fuel cell; solid oxide fuel; phosphoric acid fuel cells.
The best known early fuel cell experiments were performed in 1842 by William R. Grove (1811-1896). Grove was most probably the one, who built the first real fuel cell.
Hydrogen-oxygen fuel cell
A common type of fuel cell example is hydrogen/oxygen cell. In 1839, Grove first described the hydrogen-oxygen fuel cell.
In this type of fuel cell, hydrogen and oxygen combine electrochemically to produce electricity. It consists of two electrodes and an electrolyte. The two electrodes are made up of porous graphite (mixed with nickel powder). The electrolyte used is KOH solution.
Hydrogen and Oxygen gases are bubbled through the anode and cathode compartment respectively. Hydrogen is oxidized at the anode where as oxygen gets reduced at the cathode.
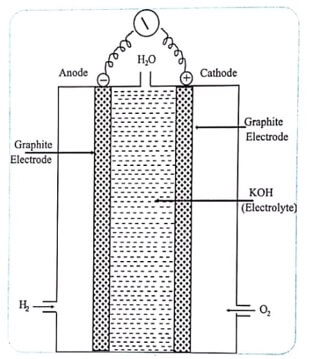
Fig (1) Hydrogen – oxygen fuel cell
Anode: 2H2(g) + 4OH– → 4H2O(1) + 4e–
Cathode: O2(g) + 2H2O +4e– → 4OH–
The overall cell reaction is thus given by
2H2(g) +O2(g) → 2H2O(1)
Generally large number of these cells are stacked together in series to make a battery called fuel cell battery or fuel battery.
Advantages and disadvantages of fuel cells
| Advantages | Disadvantages |
| 1. It has high efficiency. | 1. The cost is very high. |
| 2. It is easy to maintain. | 2. It needs to be stored in big tanks. |
| 3. It does not produce any harmful exhaust. | 3. It is difficult to predict the life time of fuel cells accurately. |
| 4. It is fuel-efficient. | 4. High cost of H2 gas. |
| 5. Water produced from hydrogen-oxygen fuel cells can be used for drinking purpose. | 5. Lack of infrastructure for distributing hydrogen. |
| 6. It can be used as a source in space flights. | – |
| 7. No noise and thermal pollution | – |
Applications of fuel cells
- Fuel cells are ideal for power generation.
- They are used for powering buses, boats and trains.
- Hospitals use fuel cells to provide electricity.
- All major auto makers are working to commercialise a fuel cell car.
- Fuel cells are used in smart phones, laptops and tablets.
| Read More Topics |
| Write a note on synthetic abrasives |
| Nickel cadmium NICAD battery |
| An overview of primary alkaline battery |





