Sympathomimetic drugs stimulate the sympathetic or adrenergic nerve, directly by mimicking the action of NE or indirectly by stimulating the release of NE.
STRUCTURE ACTIVITY RELATIONSHIP
The sympathomimetic drugs may be divided into catechol amines.
- All the catechol amine possesses catechol nucleus.
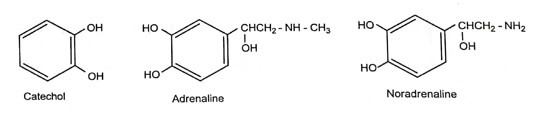
- Non catecholamine consists of a benzene ring and an ethylamine side chain.

(i) β-phenyl ethylamine type of Adrenergic Agonist
- β-phenyl ethylamine is the parent structure for many sympathomimetic drugs. The modifications of β-phenylethylamine influence not only the mechanism of action, receptor selectivity, but also their absorption, oral activity, metabolism and duration of action.
ii) The basic structure required for OptimalAgonist Activity
- Aromatic ring with amino side chain separated by two carbon
- Hydroxyl group at β-carbon of the side chain
- Primary or secondary amino group
iii) Aromatic Ring
- The presence of -OH group in the 3’ and 4’ position (Catechol) of the benzene ring show maximum α and β-adrenergic activity. But poor oral availability due to rapid metabolism by COMT.
- Removal or replacement with hydroxy methyl group of 3’ and 4’ -OH group increases the oral bioavailability and duration of activity. eg.Albuterol.
- The lack of -OH group in the aromatic ring and β-carbon of the side chain increases the CNS activity and decreases adrenergic activity. eg. Amphetamine, Methamphetamine.
- When both the -OH is removed potency of the compound reduces.eg. Phenyl epinephrine is less potent than adrenaline.
iv) Ethyl amine Substitution
- For optimal activity the amino group should be separated from aromatic ring by 2 -carbon atom.
- The presence of -OH group in β-carbon enhances agonist activity at both α and β receptor and decreases the CNS activity.
- Substitution in C1 and C2 results in optical isomers; 1R,2S isomer seems to be correct configuration for direct acting agonist.
- Substitution in the β-carbon decreases CNS activity and increases adrenergic activity. For example, Epinephrine is less potent than Methamphetamine as a CNS stimulant, but more potent vasoconstrictor and bronchodilator.
v) Amino Substitution
- The presence of amino group in phenyl ethyl amine is important for direct agonist activity.
- The amine is normally ionized at physiological and for optimal activity the pKa should be between 8.5-10.0.
- 1°and 2° amine have good adrenergic activity than 3° and quaternary ammonium salt.
- As the size of the alkyl group in the amino group increases, α-receptor agonist activity decreases and β-agonist activity increases

Imidazoline type of α- Adrenergic Agonist
- A second chemical class of α-agonists is imidazolines. These agents are selective α-Adrenergic agonist vasoconstrictor. eg. Naphazoline, Antazoline, Tetrahydrozoline, Xylomethazoline. They are selective for either α1 or α2 – receptors.
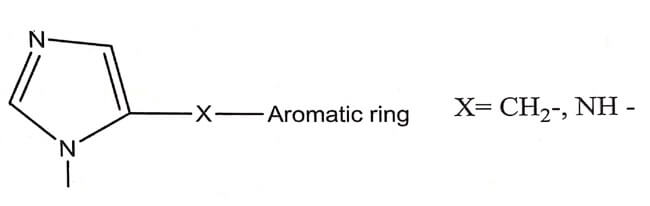
- Structurally , most imidazolines have their heterocylic imidazoline nucleus linked to a substituted aromatic moiety via some type of bridging unit. The optimum bridging unit (X) is usually a single methylene group or amino group.
- Substituting in the aromatic ring enhances the agonist activity.
| Read More Topics |
| Cholinomimetic or parasympathomimetic agents |
| Morphine and its related compounds |
| Application of classical Bioisosteres in drug design |