It is impossible to know the absolute values of the single electrode potential.
It is because a single electrode constitutes only a half- cell. A half-cell would not lose or gain electrons by itself. The loss or gain of electrons can take place only in a complete circuit containing two half- cells connected to each other.
Therefore, the electrode potential can only be measured by using some electrodes known as reference electrodes. The reference electrode most commonly used is the Standard Hydrogen Electrode (SHE). The potential of SHE is taken as zero. The emf of the cell is measured.
Reference Electrode
An electrode whose electrode potential is accurately known or whose electrode potential has been arbitrarily fixed is called a reference electrode.
Measurement of Single Electrode Potential
For measurement of standard electrode potential, a cell is set up in which one-half of the cell consists of metal and its ions at 1°M concentration and the other half of the cell consists of standard hydrogen electrode.
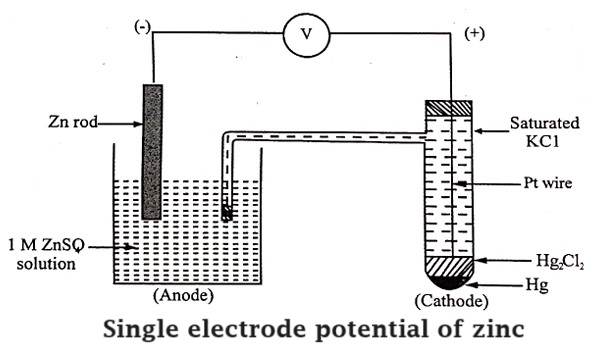
Measurement of single electrode potential of a zinc.
To determine single electrode potential of zinc, the zinc electrode is dipped in 1°M zinc sulphate solution. The half cell is coupled with SHE. The two half- cells are connected through a salt bridge.
Zinc electrode when combined with SHE is oxidised to Zn2+ ions. In other words, the zinc electrode pushes electrons into the external circuit and forms the anode.
The voltmeter reading gives the electrode potential of zinc, as SHE potential is assigned zero. Here, zinc acts as anode.
Zn → Zn2+ + 2e-
It is said to possess a negative value of reduction electrode potential (−0.76°V).
| Read More Topics |
| Definition and construction of electrochemical cell |
| Definition and origin of electrode potential |
| Introduction of electrochemistry |





