Nuclear chain reaction is defined as a fission reaction where the neutrons from a previous step continue to propagate and repeat the reaction.
We know that U235 when bombarded hit by a neutron undergoes the following fission reaction:
92U235 + 0n1 → 56Ba140 + 36Kr93 + 30n1 + Energy
In this reaction three neutrons are produced. These three neutrons in turn may cause fission in three U235 nuclei producing 9 neutrons along with lots of energy.
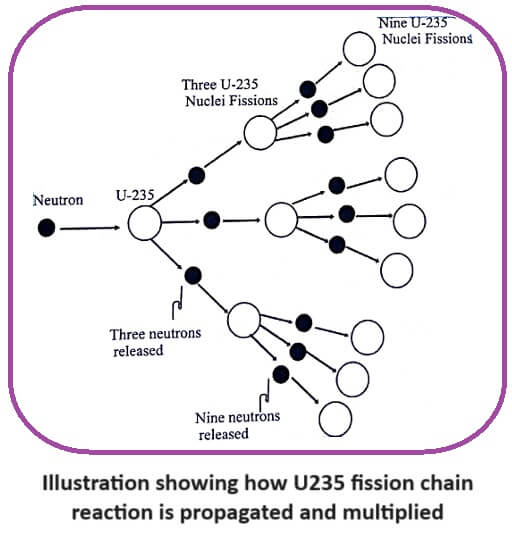
These nine neutrons in turn cause the fission in nine more U235 nuclei, producing 27 neutrons and get greater amount of energy. Hence the fission of U nucleus goes on like an unending chain.
This process of propagation of the reaction by threes times at each fission is referred to as chain reaction (Fig).
A chain reaction will continue until the splitting of the U235 nuclei takes place. It is observed that not all the neutrons released in the reactions are used up in propagating the chain reaction. Some neutrons may miss their target and some escape into air and thus are lost.
Criteria:
Thus, for a chain reaction to occur,
- The size of the fissionable material should be large enough to capture the neutron.
- If the size is too small, more neutrons will escape from its surface thereby breaking the chain.
The minimum mass of fissionable material required to sustain or continue a chain reaction is called critical mass. The critical mass varies for each reaction. For U235 the critical mass is 10°kg.
Sub-critical mass is a mass less than its critical mass.
Super-critical mass is a mass greater than its critical mass.
The chain reaction can be controlled by absorbing a desired number of neutrons (using some suitable materials like Cd, B, steel) so that on an average only one neutron remains available for carrying out further fission. Such a reaction is called controlled chain reaction. The energy released in such a controlled chain reaction does not go out of control.
This controlled chain reaction is used in nuclear reactors.
| Read More Topics |
| Characteristics of nuclear fusion |
| What are the factors influencing the corrosion? |
| Types of electrochemical corrosion |





