Depending on the separation between the centre of gravity of positive charges and the centre of gravity of negative charges, the dielectrics are classified as polar (dipole) dielectrics and non-polar (neutral) dielectrics.
Polar molecules
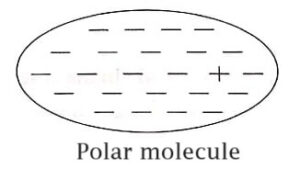
The polar molecule is one in which it will not have centre of symmetry. Here the ‘centres’ of positive and negative charges will not coincide and hence it possess a net dipole moment in it.
So, the polar molecules is thus an electric dipole and has an intrinsic (permanent) dipole moment.
Examples: H2O,N2O,HCL,NH3,CO,CH3 OH etc.
Effect of electric field
In the absence of electric field : In the absence of electric field itself, the polar molecules posses some dipole moment. But, since these dipoles are randomly oriented they cancel each other and the net dipole moment will be very very less (approximately zero).
In the presence of electric field: Now, when an external electric field, is applied to the polar molecules, the dipoles will align themselves parallel to the field direction and produces a net dipole moment.
Non-Polar molecules
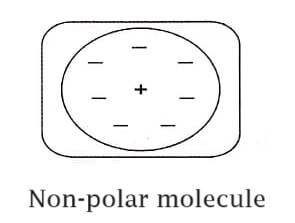
The nonpolar molecule is one in which it posses centre of symmetry and hence the ‘centres’ of positive and negative charges coincides. Therefore the net charge and net dipole moment of these molecules will be zero and hence these non-polar molecules will not posses any dipole moment in it.
Examples: N2, H2, O2, CH4, CO2 etc.
Effect of electric field
In the absence of electric field : In the absence of electric field, the positive and negative charges are so close to each other that their action becomes neutralized.
In the presence of electric field: When a non-polar molecule is placed in an external electric field, a force is exerted on each charged particle within the molecule. (i.e.) the positive particles are pushed along the field direction and the negative charges are pushed opposite to the field direction.
Hence, the displacement of these charges results in the creation of electric dipole. Since, the positive and negative charges are separated by some distance from their equilibrium positions, creating a dipole and therefore a net dipole moment will be produced in non-polar molecules.
Differences Between Polar and Non-Polar Molecules
| S.No | Polar Molecules | Non-Polar Molecules |
| 1. | Polar molecules have permanent dipole moments even in the absence of an applied field. | Non-polar molecules do not have permanent dipole moments. |
| 2. | Polarization of polar molecules is highly temperature dependent. | Polarization of this kind of molecules is independent of temperature. |
| 3. | These molecules do not have symmertrical structure and they do not have centre of symmetry. | These molecules have symmetrical structure and they have centre of symmetry. |
| 4. | For this kind of molecules, there is absorption or emission in the infrared range.
Examples: CHCI3, H2O, HCl |
For these molecules, there is no absorption or emission in the range of infrared.
Examples: CCL4, CO2, H2 |
| Read More Topics |
| Classification of dielectric materials |
| Applications of superconducting materials |
| Temperature dependence of resistance |