Since the atoms in the metallic glasses are disordered, they have some peculiar properties as follows.
a) Structural properties
- Metallic glass have tetrahedral closely packed (TCP) structure rather than hexagonal closely packed (HCP) structure.
- They do not have any crystal defects such as grain boundaries, dislocations, etc.
b) Mechanical (or) General properties
- The metallic glasses are very strong in nature.
- They have high corrosion resistance.
- They possess high brittle, ductility etc.
c) Electrical properties
- Metallic glasses have high electrical resistance (100μw−cm).
- They have high electrical resistivity which leads to very low eddy current losses.
- At low temperature, resistivity is minimum, below which it varies as a logarithmic function of temperature.
- At very low temperature, thermal expansion co-efficient is negative.
d) Chemical Properties
- Highly resistant to corrosion.
- Highly reactive and stable.
- They act as catalyst.
e) Magnetic properties
- They exhibit high saturation magnetization.
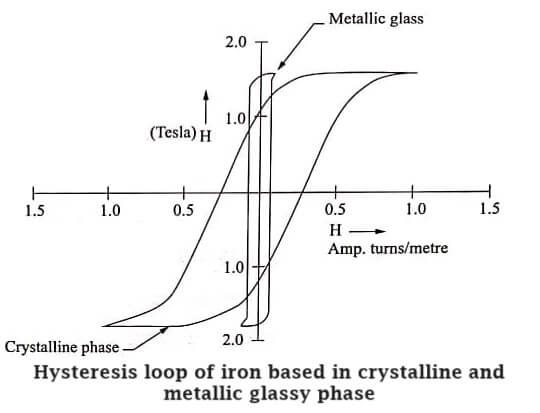
- Metallic glasses are soft magnetic materials so they can be easily magnetized and demagnetized.
- They have high magnetic permeability and exhibit ferromagnetism.
Applications of Metallic Glasses
- Metallic glass are ferromagnetic. So these are used in tape recorder heads, cores of high power transformers and magnetic shields. Example:Fe75P15C10,Fe24,Zr76,Ni60,Nb40
- They have higher workability. They can be easily bent without breaking. Hence these are useful in making different kinds of springs.
- Metallic glasses are very useful in the production of very high magnetic fields.
- Metallic glasses have high electrical resistance with nearly zero temperature coefficient of resistance. Hence, they are used to make accurate standard resistances, computer memories, magnet resistance sensors and cryo-thermometers.
- Since the random ordering does not have any lattice defects, they posses high tensile strength. Its hardness is also very high. Hence they are used as reinforcing elements in concrete, plastic or rubber.
- Since they are not affected by nuclear radiation, they are used in making containers for radioactive waste disposal.
- Since they have very high corrosion resistance, Cr and P based metallic glasses are used in marine cables, inner surface of reactor vessels, orthopaedical implants and surgical clips.
- Metallic glasses are used as transformer core material in high power transformers. It found to improve the efficiency of power distribution in transformers.
| Read More Topics |
| Semiconducting materials – solved problems |
| Conducting materials – solved problems |
| Dielectric materials – solved problems |





