These receptors possess a subunit along with enzymatic property or bind a JAK (Janus Kinase) enzyme on activation. The outer face of plasma membrane carry binding site for agonist and the inner face has catalytic site (figure). A single transmembrane stretch of peptide chain interconnects these two domains. The two primary subgroups of these receptors are:
- Receptors having intrinsic activity.
- Receptors having no intrinsic enzymatic activity, but bind a JAK-STAT kinase on activation.
Both these mechanisms are described here:
Intrinsic Enzyme Receptors : The intracellular domains are either protein kinase or guanylyl cyclase. The protein kinase usually phosphorylates tyrosine residues present on substrate proteins (figure A ), like insulin, Epidermal Growth Factor (EGF), Nerve Growth Factor (NGF) receptors. In some cases, protein kinase works on serine or threonine.
The kinase is inactive in monomeric state. When Agonist binds, dimerisation of receptor molecules induces and the kinase gets activated. Kinase in turn autophosphorylate tyrosine residues that elevate their affinity for substrate proteins attachment and making forward cascade of tyrosine phosphorylation.
Phosphorylation of certain proteins (carry an SH2 domain) triggered by intracellular events. The dimerisation of these receptors promotes receptor internalisation, lysosomal degradation and down regulation.
The enzyme participating could be Guanylyl Cyclase (GC), like in the Atrial Natriuretic Peptide (ANP). Receptor agonist activation promotes cytosolic cGMP as a second messenger that activates cGMP-dependent protein kinase and modifies cellular activity.
JAK-STAT-Kinase Binding Receptors : The primary feature of these receptors is absence of intrinsic catalytic domain. When agonist induces dimerisation it changes the conformation of intracellular domain. This raises the affinity towards cytosolic tyrosine protein kinase JAK. Upon binding, activation of JAK occurs and tyrosine residues on the receptor get phosphorylated. This now attaches to next free protein STAT (Signal Transducer and Activator of Transcription) which also gets phosphorylated further by JAK.
Pairs of phosphorylates STAT dimerises and move to the nucleus regulating gene transcription. This produces a biological response. Many cytokines, growth hormone, interferons, etc., perform using these receptors.
The responses are transduced by enzyme-linked receptors in few minutes to hours.
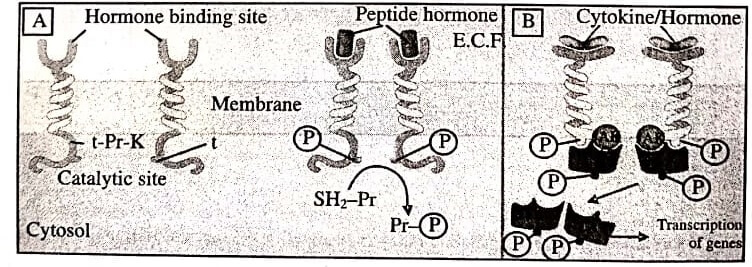
A) Intrinsic Tyrosine Protein Kinases Receptor. On binding the peptide hormone to the extracellular domains, the monomeric receptors move laterally in the membrane and form dimers. Dimerisation activates tyrosine-protein kinase (t-Pr-K) activity of the intracellular domains so that they phosphorylate tyrosine (t) residues on each other, as well as on several SH2 domain substrate proteins (SH2−Pr). The phosphorylated substance proteins then perform downstream signalling function.
B) JAK-STAT Kinase Binding Receptor. The intracellular domain of these receptors lacks intrinsic protein kinase activity. Signal molecules binding to the extracellular domain induces receptor dimerisation which activates the intracellular domain to bind free moving JAK (Janus Kinase) moiecules. The activated JAK phosphorylate tyrosine residues on the receptor which then binds another protein STAT (Signal Transducer and Activator of Transcription). Tyrosine residues of STAT also get phosphorylated by JAK. The phosphorylated STAT dimerise, dissociate, from the receptor and move to the nucleus to regulate transcription of target genes.
| Read More Topics |
| G -Protein coupled receptors |
| Factors affecting drug receptor interaction |
| Plasma protein binding of drug |
| Introduction and nature of drugs |





