There are two types of electrochemical Corrosion:
- Galvanic corrosion
- Differential aeration or Concentration cell corrosion
Galvanic corrosion
When two dissimilar metals are in contact with each other on the presence of aqueous solution or moisture, galvanic corrosion occurs.
The more active metal (with high negative electrode potential and higher in the electrode potential and higher in the electrochemical series) acts as anode and the other metal lower in the electrochemical series acts as cathode.

For example,
In the Zn−Fe galvanic cell, Zn behaves as anode where oxidation and corrosion occurs and Fe behaves as cathode.
But in Fe−Cu galvanic cell, Fe acts as anode and undergoes corrosion and Cu acts as cathode.
Examples:
- Steel pipe connected to copper.
- Steel screw in a brass hinge.
- Lead-antimony solder around copper wire.
The rate of galvanic corrosion is directly proportional to the area of the cathode. This type of corrosion is maximum near the junction of the two metals.
Galvanic corrosion can be prevented or minimised by
- Fixing and insulating material between the two metals.
- Making the cathode smaller and anode larger in area.
- Coupling metals close together as possible in the electrochemical series.
- Using inhibitors to reduce aggressiveness of the environment.
Galvanic corrosion has several beneficial applications, e.g., dry cells, cathodic protection etc.,
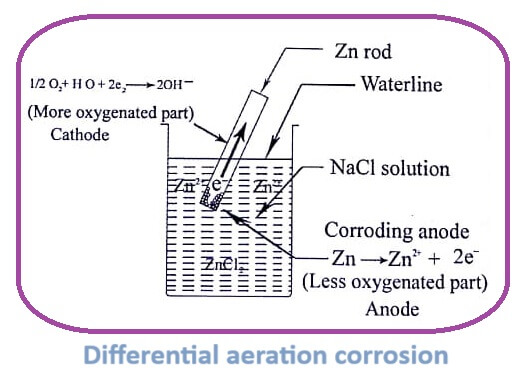
Differential aeration corrosion
This type of corrosion occurs when a metal is exposed to an electrolyte with varying amount of oxygen.
The metal part immersed in a liquid or an electrolyte or partially buried in soil is less aerated (i.e. less oxygenated) and act as anode. The rest of the metal parts which are exposed to atmospheric oxygen act as cathode.
At anode (less oxygenated part),
Corrosion occurs
M → Mn+ + Ne–
At cathode (more oxygenated part),
1 /2 O2 + H2 O + 2e– → 2OH–
Examples of differential aeration corrosion are:
- Waterline corrosion,
- Pitting corrosion,
- Crevice corrosion and
- Pipeline corrosion.
1. Waterline corrosion
Let us consider metal tank partially filled up with water. The metal area above water line is exposed to higher concentration of oxygen (cathode) than the metal below water level. The metal less exposed to O2 acts as anode and corrodes. This is called water line corrosion.
This is a serious problem for ocean-going ships.

Prevention of waterline corrosion
- Avoid water stagnation in tanks.
- Protecting coating is used to reduce the corrosion.
- Lining inside the tank by chemical-proof sheets.
2. Pitting corrosion
Sometimes corrosion may be concentrated at some places and at other places it may be less. Thus, pits or holes are formed.
The presence of impurities such as sand, dust etc., present on the metal surface leads to pitting. It starts with small crevices (pits).
Let us consider a drop of water resting on a metal surface. The area covered by the drop or dirt acts as anode and corrodes. The uncovered area exposed to air or O2 acts as cathode.
As the anodic area is small compared to the cathodic area, more and more metal are removed at the same spot. Thus a small hole is formed on the surface of the metal. This type of intense corrosion is called pitting.

Prevention of pitting corrosion
- Maintaining clean surface.
- Preventing stagnant conditions.
- Applying cathodic protection.
- Using alloys with higher resistance to pitting.
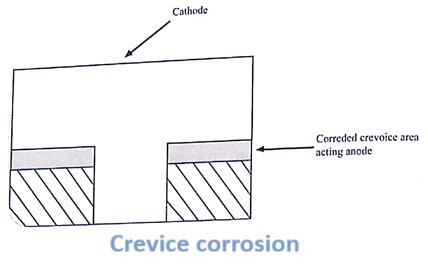
3. Crevice corrosion
If a crevice or crack between two different metallic or nonmetallic materials, e.g., bolts, nuts, rivets etc., is in contact with liquid, the crevice becomes the anodic part and gets corroded. This is because the crevice area lacks oxygen. The exposed area having high oxygen acts as cathode.
4. Pipeline corrosion

Buried pipelines or cables passing from one type of soil (clay, less aerated) to another type of soil ( sand, more aerated) may get corroded due to differential aeration. This type of corrosion occurring in pipelines is called pipeline corrosion.
5. Corrosion on wire-fence
In a wire fence, where the cross wires are less aerated than the rest of the fence, corrosion takes place at the wire crossings, which become anodic and enhance the electrochemical corrosion.

| Read More Topics |
| What are the 2 types of corrosion? |
| Corrosion in day to day life |
| Electrochemistry – Solved problems |





