The water used in industries should be free from impurities, hardness corrosive agents. If these salts are not removed properly, problems like scale and sludge formation, corrosion etc., occur in the boiler.
The process of removing hardness producing substances from the water is called ‘softening of water’.
Types of softening of water processes
- External treatment;
- Internal treatment.
External treatment or external conditioning
The process of treatment of water before supplying into the boiler is known as external treatment.
The main purpose of external treatment is to carry out efficient softening of water. The water should be free from all types of impurities. This can be achieved by:
- zeolite or permutit process and
- demineralisation or deionisation process.
Demineralisation process or ion-exchange process
Ion-exchange methods for the purification of water help in the removal of all soluble minerals (both cations and anions) without distillation.
In this process, hardness producing cations (like Ca2+,Mg2+ ) and anions (like Cl–,SO42- ) are removed by ion-exchange resins.
Ion-exchange resins used in ion-exchange process are big organic molecules, i.e., they are polymers of high molecular weights and are in the form of porous grains.
There are two types of resins:
- Cation-exchange resins: These contain carboxy (-COOH) groups. The electropositive ions (cations) present in water are exchanged by these resins for their H+ ions.
- Anion-exchange resins: These contain basic groups such as hydroxyl (-OH) groups. The electronegative ions (anions) present in water are exchanged by these resins for their -OH– ions.
Process
Each type of resin is taken in a separate tank.
The hard water is first passed through a tank containing the cation-exchange resin. Cations like Ca2+,Mg2+ and Na2+ present in water are exchanged by the cation-exchange resin for its H+ ions.
2RCOOH + Mg2+ → (RCOO)2 Mg + 2H+
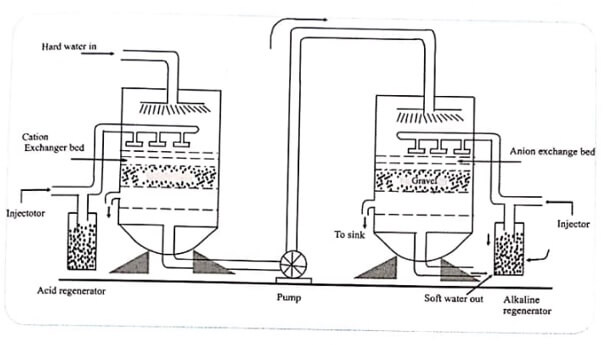
Fig 1.1 Demineralisation of Water
The water containing H+ ions passes through the second tank which contains the anion-exchange resin.
The anions like Cl–,SO42- and HCO3– present in water are exchanged by the anion-exchange resin for its OH– ions.
2(RNH3)OH + SO4– → (RNH3)2SO4 + 2OH–
OH– ions released in the anion-exchange tank combine with the H+ ions which are already present in water to form unionised water molecules.
H+ + OH– → H2O
Thus, the water coming out of the second tank contains practically no ions. It is almost like distilled water. Thus, ion-free water is called “deionised water” or ” demineralised water”.
Regeneration
The resin in the first tank, when exhausted, may be regenerated by passing dilute sulphuric acid through the tank.
The resin in the second tank, when exhausted, may be regenerated by passing moderately concentrated solution of sodium hydroxide.
RSO4 + 2NaOH → R(OH)2 + 2Na2SO4
Advantages and disadvantages
| S. No | Advantages | Disadvantages |
| 1. | The process can be used to soften highly acidic or alkaline waters. | The equipment is costly. |
| 2. | Highly economical. | More expensive chemicals are needed. |
| 3. | It does not cause foaming and priming problems when used in boilers. | Turbid water cannot be treated and hence reduces the output of the process. |
| 4. | It produces water of very low hardness (2 ppm). Hence, it is very good for use in high-pressure boilers. |
Zeolite process
Zeolites are hydrated sodium aluminosilicates, which are capable of exchanging reversibly their sodium ions for hardness producing ions in water.
- Zeolites are also known as permutits. Hence, zeolite process is called as permutit process.
- Chemical structure of sodium zeolite is,
Na2O.Al2O3.×SiO2.yH20.
where x = 2-10 and y = 2-6.
There are two kinds of zeolites:
(i) natural zeolites;
(ii) synthetic zeolites.
Difference between natural and synthetic zeolites
| S.No | Natural zeolites | Synthetic zeolites |
| 1 | They occur in nature
E.g., Natrolite, Na2O.Al2O3.4SiO2.2H2O. |
They may be prepared by heating solutions of sodium silicate, aluminium sulphate and sodium aluminate. |
| 2 | Non-porous in nature. | They are porous and process gel structure. |
| 3 | Natural zeolites possess lesser exchange capacity per unit weight than the synthetic zeolites. | Synthetic zeolites possess higher exchange capacity per unit weight than natural zeolites. |
Principle
The water containing hardness producing substances like calcium (Ca++)and magnesium (Mg++)etc., are passed over the bed of sodium zeolite.
The following reactions take place. Calcium and magnesium ions are exchanged for sodium ions as shown below:
CaCl2 + Na2Ze → CaZe + 2NaCl
MgSO4 + Na2Ze → MgZe + Na2 SO4
Mg(HCO3)2 + Na2 Ze → MgZe + 2 NaHCO3
Thus, sodium zeolite is converted into calcium and magnesium zeolites which are called exhausted zeolites.
Now, water becomes free from calcium and magnesium salts and gets richer in sodium salts.
In this process, zeolite acts as a ‘sodium exchanger’.
Process
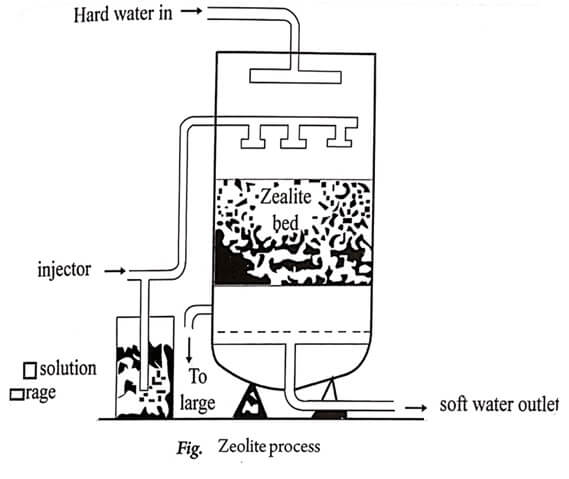
The process is carried out with the help of ‘zeolite softener’, which is shown in the Fig.
Hard water is passed at a specific rate through the bed of zeolite, which is kept in a long cylinder.
The hardness causing ions Ca++ and Mg++ are retained by zeolites as calcium zeolite ( CaZe) and magnesium zeolite ( MgZe).
Water free from hardness producing ions are collected from the zeolite softener.
Regeneration
In the above-mentioned process, sodium zeolite is completely converted into calcium and magnesium zeolites and in the long run zeolites lose its originality and get exhausted completely.
The exhausted zeolites are regenerated by treating it with 10% of brine solution (concentrated NaCl solution).
Advantages and disadvantages of zeolite process
| Advantages | Disadvantages |
| It removes hardness almost completely. | The method replaces only Ca++ and Mg++ ions by sodium ions. Acidic ions like HCO–3 and CO–3 remain in the outcoming water. |
| The process adjusts itself for different hardness of incoming water. | Presence of acidic ions like HCO3– and CO3– in the water in turn produces CO2 and NaOH. So, this water if used in boilers produces caustic embrittlement. |
| Time requirement is less for preparing the softened water. | Any turbidity in water affects the nature of the zeolite bed. Presence of turbidity will clog the pores of the zeolite bed, thereby making it inactive. |
| The equipment occupies less space. | Presence of mineral acid in water, will destroy the zeolite bed. |
| Its maintenance and operation are easy. | Presence of ions like Fe++ and Mn++ in the raw water produces iron zeolite and manganese zeolite, Hence, Fe++ and Mn++ ions should be removed first before feeding into the zeolite softener. |
Difference between zeolite process and demineralisation process
| S.No | Zeolite process | Demineralisation or ion-exchange or deionisation process |
| 1 | This is an ion-exchange process where exchange process is carried out by zeolite. | This is also ion-exchange process but the exchange process is carried out by ion-exchange resins. |
| 2 | Only cations can be removed by this method. | Almost all types of ions (both cations and anions) can be removed by this method. |
| 3 | The water obtained by this method causes foaming and priming problems, when used in boilers. | The water obtained this method does not cause foaming and priming problems, when used in boilers. |
| 4 | Acidic water cannot be used by this method. Because, presence of any acidic ions in water affects the nature of zeolites. | Acidic water can be processed by this method. |
| 5 | Iron containing water and turbid natured water cannot be treated by this method. | Iron containing water and turbid natured water can be treated by this method. |
[sc_fs_faq html=”true” headline=”h2″ img=”” question=”What is softening of water?” img_alt=”” css_class=””] Softening of water is the process of removing minerals, primarily calcium and magnesium ions, from hard water. This is typically done to make the water more suitable for various purposes, such as household use and industrial applications. Softened water helps prevent scaling in pipes and appliances, improves soap lathering, and reduces water spotting on dishes and fixtures. [/sc_fs_faq]
| Read More Topics |
| Requirement of boiler feed water |
| Introduction of water technology |
| Dalton’s law of partial pressures |





