The method of western blot was discovered by George Stark at Stanford, and the name western blot was laid down by W. Neal Burnette. This method, also known as immunoblot or protein blot, detects specific proteins in a sample of tissue homogenate or extract.
Western blotting is a qualitative and semi-quantitative technique for protein analysis. This technique is useful in cell and molecular biology. It aids in the identification of desired protein from a mixture of proteins extracted from cells. It also facilitates evaluation of the size and amount of protein. The technique of western blotting requires SDS-PAGE.
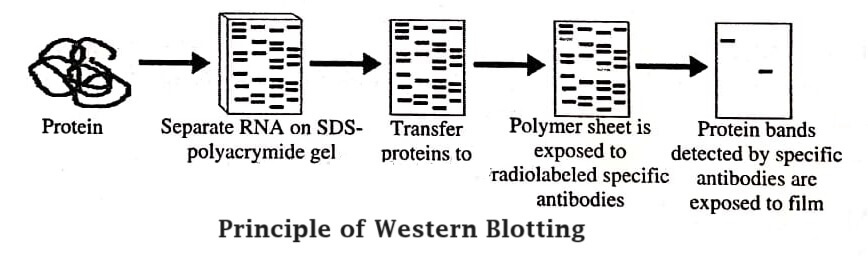
Principle of Western Blotting
Western blotting is a fast and sensitive method of assay that detects and characterises proteins. It works on immunochromatography principle, wherein separation of proteins as per their molecular weight occurs in polyacrylamide gel. The separated proteins are transferred or electro-transferred on nitrocellulose membrane, and then detected by specific primary antibody and secondary enzyme labelled antibody and substrate.
Procedure of Western Blotting
The steps involved in the procedure of western blotting are:
Tissue Preparation (Preparation of Sample Lysate): In the sample, ice cold PBS and lysis buffer (RIPA buffer that provides maximum protein yield) are added. The lysis buffer is selected based on the localisation of the desired protein. Unlike isolated cytoplasmic proteins, the solubilisation of membrane bound proteins is achieved by stronger extraction detergents.
Lysis buffer should possess protease inhibitors that avoid the degradation of the desired protein. Lysis of cells is done by incubating on ice and then applying shear pressure through pipette. Later the cell mixture is centrifuged and the pellet is discarded. The supernatant obtained is the lysate of use.
Gel Electrophoresis: The individual proteins in the obtained sample lysate are separated based on their molecular weight. The separation is achieved by using a positive electrode that attracts a negatively charged protein. This is done by introducing the prepared protein sample into a polyacrylamide gel (commercially available). Gels of fixed percentages or gradients of acrylamide are commercially available.
Higher the acrylamide percentage, smaller the pore size of the gel matrix. Hence for low molecular weight proteins, higher percentage of gels is preferred; whereas for large proteins, low percentage of gel is considered better. Gradient gels have varied range of pore size, thus are useful for proteins of all sizes.
The steps involved in gel electrophoresis are:
- The gel is prepared by inserting it in the electrophoresis apparatus and filling with running buffer.
- The wells of the gel are washed with running buffer and the chambers are added with buffer.
- The samples are loaded into the wells. A pre-stained molecular weight ladder is loaded in one well to aid in monitoring protein separation during electrophoresis and verifying protein weight in the sample later during analysis.
- The electrophoresis unit is closed and a power supply is connected. The majority of units run for 45-60 minutes at 200 volts or until the loading buffer seeps to the bottom of gel. In this time period, the negatively charged sample proteins migrate towards the positive electrode through the polyacrylamide gel matrix.
Transfer: The separated proteins from gel are transferred to a solid membrane or blot. This works on the principle of applying electric field whereby negative proteins shift towards a positive electrode. Wet or semi-dry conditions are used for this transfer.
The wet transfer method involves the following steps:
- The gel is removed from its cassette by cutting the top portion of the wells.
- The top left corner is marked to indicate gel direction.
- The gel is allowed to float in transfer buffer while preparing the transfer sandwich using a cassette, sponges, filter paper, the gel, and PVDF or nitrocellulose membrane.
- The top left corner is marked on the blotting paper to indicate blot direction.
- The membranes are incubated for 10 minutes in transfer buffer.
- A stack is prepared by placing sponge, filter paper, gel, membrane, filter paper, and sponge from the black negative cathode towards red positive anode.
- The gel or membrane should not be touched with bare hands and clean tweezers or spatula should be used, since touching the membrane can contaminate the blot. As a result, excessive background signals will appear.
- A clean roller should be employed at every layer to remove bubbles or else they will interfere with efficient protein transfer.
- The cassette is locked and placed in the transfer apparatus containing cold transfer buffer positioned from negative to positive.
- The heat development can be avoided by transferring with a cold pack in the apparatus or operating in a cold room with the spinner bar at the bottom of the chamber.
- The chamber is closed and power supply is connected.
- Transfer is done as per the manufacturer’s instructions (usually, 100 volts for third to 120 minutes is recommended).
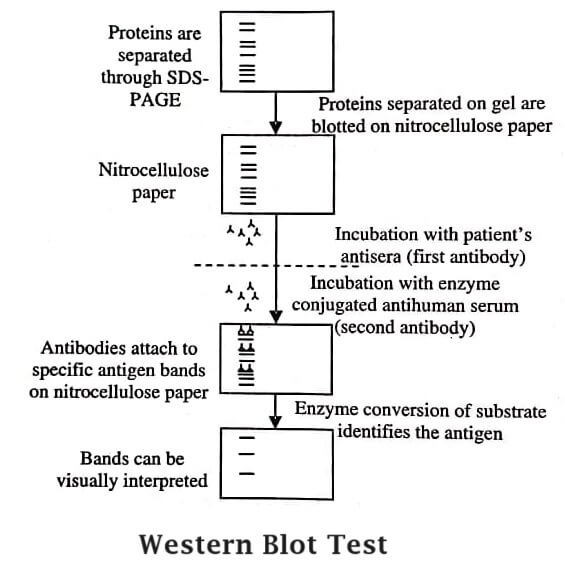
Immuno-Blotting: When protein is electrically transferred to a membrane, the blot is blocked using a protein specific primary antibody. Later, a secondary antibody is added that identifies the primary antibody.
- The immuno-blotting method involves the following steps:
- The membrane is detached from the cassette and rinsed thrice with water.
- The blot areas which do not contain protein are blocked to avoid nonspecific antibody binding and to minimise overall background signal.
- The commonly used blocking buffers are 5% non-fat dry milk or BSA in a TBS-Tween solution. Milk solution is avoided when probing with phosphor-specific antibodies to prevent high background from its endogenous phosphoprotein, casein.
- The membrane along with blocking solution is incubated for an hour at room temperature with slight agitation.
- The blocking solution is decanted and washed for 5 minutes with TBS tween.
- Before adding the primary antibody, it is diluted with a blocking buffer (in specified concentration) and incubated overnight with gentle shaking at 4°C temperature.
- After incubation the primary antibody is decanted. The membrane is washed five times for five minutes each using large volumes of TBS tween with vigorous agitation to eradicate non-specific background signals.
- Thereafter, the secondary antibody is diluted in blocking solution. The membrane is incubated for an hour at room temperature at the specified concentration.
- The membrane is decanted and washed five times for five minutes each with large volumes of TBS tween followed by vigorous agitation.
Detection: In this final step, signal development is achieved by electrochemiluminescence (or ECL reaction). It is a common, sensitive and inexpensive method of detection in which HRP enzyme conjugated to the secondary is used to catalyse the ECL reaction and give out light. This emitted light is projected onto X-ray film and developed or digitised using specialised and sensitive camera.
The detection method involves the following steps:
- Equal parts of ECL reagents are mixed in 1:1 ratio as per the manufacturer’s instructions.
- The membrane is incubated for 3-5 minutes with no agitation.
- The ECL mixture is decanted after incubation, and the excess solution is wiped off from the membrane corner.
- In order to avoid drying, the membrane is wrapped in a clear plastic-like sheet protector.
- A roller is used to remove bubbles (if formed) or any excess solution.
- Without further delay, the membrane is developed.
- Manual adjustment of the exposure time is done by using film and camera systems to obtain a picture perfect western blot.
- Quantification of relative band densities is done using the commercially available software.
- Molecular weight verification can also be achieved through comparison between the band sizes and the molecular weight ladder.
Applications
The technique of western blotting has the following applications:
- It can be used to determine the size and amount of protein in the sample.
- It can be used in disease diagnosis by detecting the antibody (produced against virus or bacteria) present in serum.
- It can be used to identify defective proteins, e.g., in Prions disease.
- It is the confirmatory test for HIV as it determines the presence of anti-HIV antibody in the serum.
- It is a perfect test to determine the prevalence of Creutzfeldt-Jacob disease, Lyme disease, Hepatitis B, and Herpes.
| Read More Topics |
| Large scale production of MAbs |
| Genetic organisation of eukaryotes and prokaryotes |
| Major histocompatibility complex (MHC) |