ABRASIVES
Abrasives play a commendable role in our daily life and industry. For example, a house wife uses an abrasive stone to keep her kitchen knives sharp, a dentist uses an abrasive powder when he cleans his teeth and tiny abrasive wheels to smooth down fillings.
Abrasives are hard substances used for shaping, grinding and polishing the surfaces.
Characteristics of abrasives
Abrasives have the following characteristics:
- Hardness of an abrasive is a most important quality. It is measured by Mohs scale.
- They also posses high melting point and chemical inertness.
- They are widely used in the form of granules or powder.
- Some examples of abrasives are diamond, corundum, boron carbide (B4C), carborundum (SiC) etc.
Hardness of an abrasive
The capacity of an abrasive to grind another surface is known as ‘Hardness of an abrasive’. Hard abrasive has high scratch power.
Mohs, a mineralogist, has suggested a scale for determining the hardness of abrasive substances known as Mohs scale. The hardness of hardest material diamond is taken as 10 in the scale.
Mohs scale of hardness
Hardness is the most important property of an abrasive. It is the ability or capacity of an abrasive to grind away another substance.
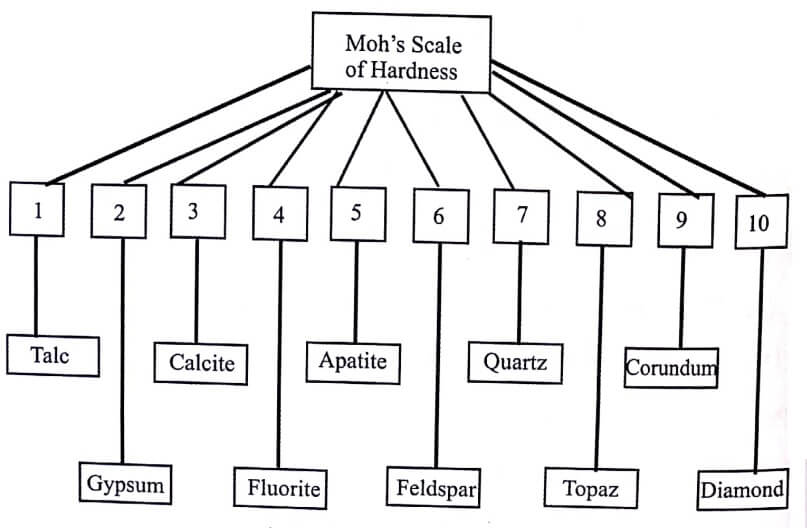
The Mohs scale is given as follows:
| Name of the abrasives | Chemical formula | Mohs number |
| Talc | 3MgO 4SiO2 H2O | 1 |
| Gypsum | CaSO4⋅2H2O | 2 |
| Calcite | CaCO3 | 3 |
| Fluorite | CaF2 | 4 |
| Apatite | CaF2 3Ca3(PO4)2 | 5 |
| Feldspar | K2O Al2O3 6H2O | 6 |
| Quartz | SiO2 | 7 |
| Topaz | AlF3 SiO2 | 8 |
| Corundum | Al2 O3 | 9 |
| Diamond | C | 10 |
The hardness of any material on Mohs scale therefore falls between 1 and 10 as shown in Table.(1) Abrasives having their hardness in the range 1-4 in Mohs scale are called soft abrasives.
Abrasive power: The strength of an abrasive to grind away a surface is known as abrasive power. It depends upon the hardness, toughness and refractoriness of an abrasive.
Classification of Abrasives
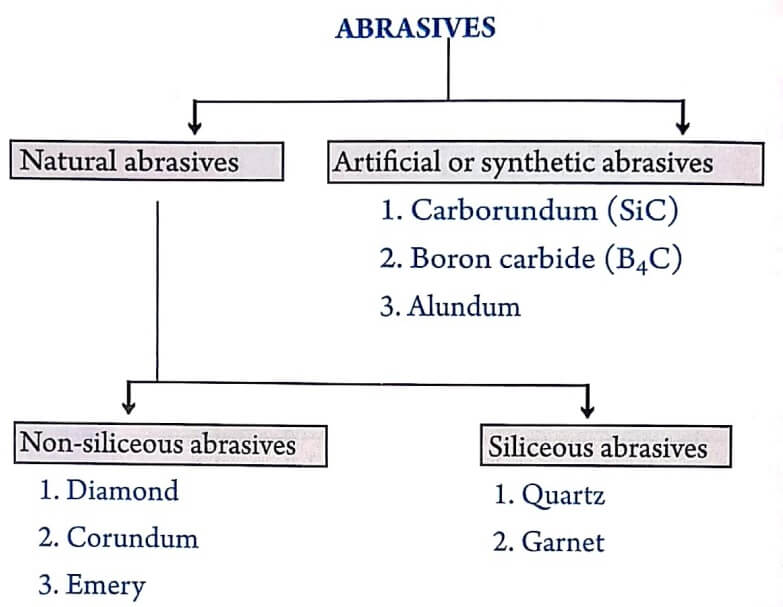
| Natural abrasives | Artificial abrasives |
| They occur in nature. | They are synthesised on a commercial scale. Hence, they are also called synthetic abrasives. |
| Some of the example of natural abrasives are diamond, emery, corundum, quartz etc. | Some of the example of this type are carborundum or silicon carbide (SiC) and Boron carbide (B4C ) etc. |
| They do not contain silica and hence called as non-siliceous abrasives. | They contain silica and hence called as siliceous abrasives. |
Natural abrasives
Natural abrasives are further classified as:
(i) Non-siliceous natural abrasives
- Diamond occurs in nature.
- It consists of crystalline carbon.
- It is the hardest among abrasives.
Uses
- It is used for cutting, grinding and polishing the surfaces. Diamond is also used as a rock driller and as a rock-cutting saw.
- Corundum is crystallised aluminium oxide (Al2O3).
- It comes next to diamond in hardness.
Uses
- It is used as an abrasive for grinding glass, gems, metals etc.
- It is a mixture of crystalline alumina and magnetite (Fe3O4) with a little amount of other minerals.
- Its hardness is about 8 on Mohs scale.
- The grinding power of emery entirely depends on the amount of alumina present in it. Its true hardness depends on the nature of other ingredients present in it.
Uses
- Emery is used as a tip of the cutting and drilling tools. Artificial emery is used for polishing.
(ii) Siliceous natural abrasives
- Quartz is a pure crystalline form of silica (SiO2).
- It is brittle in nature.
- The major defect of this abrasive is its non-uniformity.
Uses
- It is used in the manufacture of sand papers, scouring powders and soaps.
- It is widely used for grinding glass because of low cost.
- Quartz is also used for grinding pigments in the manufacturing of paints.
- It is siliceous abrasive and consists of silica minerals.
- The common garnet is a complex of calcium-aluminium -iron silicate.
- Its hardness is 6.5-7.5 in Mohs scale.
Uses
- It is used in the manufacture of coated paper and cloth.
- It is widely used for polishing hard wood and glass plate.
- It is also used for polishing metal works.
| Read More Topics |
| Nickel cadmium (NICAD) battery |
| An overview of primary alkaline battery |
| What is the basic theory of chemical kinetics? |





