Water gas
- The mixture of CO and H2 is called as water gas.
- It is also called as a blue gas due to the blue colour of the flame when it is burnt. The average composition of water gas is:
- CO=41%, H2 =51%, CO2 =4% and N2 =4%
- Its calorific value is about 2,8000 k.cals/m3.
- Its flame is short but is very hot.
Manufacture of water gas
It is obtained by passing steam over red hot coke.
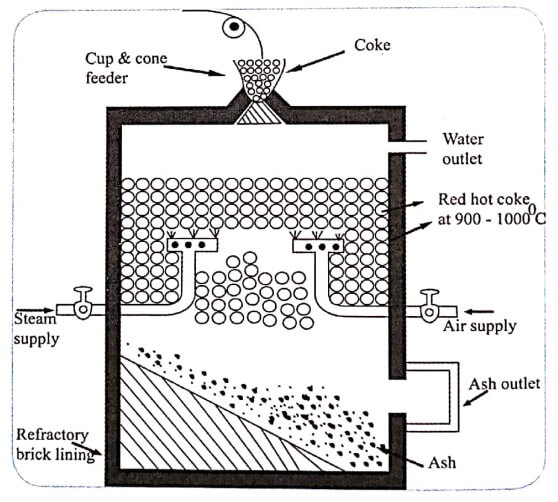
Fig 1 . Water gas Production
Reactor
Production of water gas takes place in the reactor (Fig.1), which consists of a steel vessel of about 3°m wide and 4°m high. The reactor is lined inside with fired bricks. Provisions are made for charging the coal or coke through the cup and cone arrangement fixed at the top of the reactor. Arrangements are made for the removal of ash at the bottom of the reactor.
Reaction
Steam along with little air is passed through red hot coke, maintained at about 1000°C in the reactor.
Step :1
Steam thus supplied reacts with red hot coke, to produce carbon monoxide and hydrogen.
C + H2O → CO + H2 -29kcal Endothermic reaction
Due to the endothermic nature of the reaction, the temperature of the bed falls.
Step : 2
Supply of the steam is cut off. Air is blown in and the following exothermic reaction takes place.
C + O2 → CO2 + 97°kcal
2C + O2 → 2CO + 59°kcal Exothermic reaction
The steam run and air blow are repeated alternatively in order to maintain the optimum temperature of the coke or coal bed at 1000°C. Water gas thus obtained is collected through the outlet of the reactor.
Uses:
- It is used as a furnace fuel.
- It is used as a source of hydrogen for ammonia synthesis.
- It is used to synthesis gasoline in Fischer-Tropsch process.
- It is used in the manufacture of methyl alcohol.
| Read More Topics |
| Give the composition and uses of producer gas |
| Advantages and disadvantages of gaseous fuels |
| Fraction by distillation of crude petroleum |





