Definition : Measure of tendency of a metallic electrode to lose or gain electrons when it is in contact with its own ions in solution is called electrode potential.
When an electrode, say a metal, is immersed in a solution of its ions, then there can be two possibilities:
(i) Mn+ ions may collide with the electrode, gain electrons and get converted into metal atoms (i.e., the ions are reduced): Mn+ ne– → M (reduction)
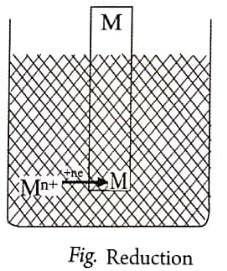
(ii) M atoms on the electrode may lose electrons to the electrode and become Mn+ ions and dissolve in the solution (i.e., oxidation occurs): M → Mn+ ne– (oxidation)
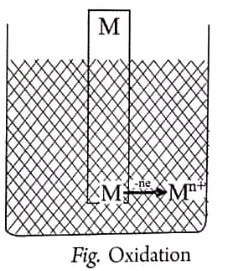
Thus, when a metal electrode is immersed in a solution of its ions, a potential difference established between the metal (or electrode) and its solution is known as an electrode potential.
Origin of electrode potential
When a metal is placed in a salt solution, a dynamic equilibrium is developed between the electrode and the solution, because of the positive (+ve) and negative (-ve) charges developed on the metal, attracts the +vely or-vely charged free ions in the solution.
Due to this attraction, the +ve and the -ve ions remain very close to the metal. This layer of positive (+ve) and negative (-ve) ions, which are formed all around the metal is called HELMHOLTZ ELECTRICAL DOUBLE LAYER.
Due to the formation of this double layer, a potential difference is set up between the metal and the solution. At equilibrium, this potential difference becomes a constant value.
The equilibrium potential difference thus established is called the single electrode potentials. In other words, tendency of an electrode to lose or gain electrons, when it is in contact with own salt is called single electrode potential. It may be oxidation or reduction potential.
The tendency of an electrode to lose electrons and get oxidised is called oxidation potential. The oxidation potential is referred as; +X volt.
The tendency of an electrode to gain electrons and get reduced is called reduction potential. The reduction potential is referred as −X volt.
The rate of the electrode potential or redox reaction depends on:
- the nature of the metal;
- the concentration of metal ion in solution;
- temperature.
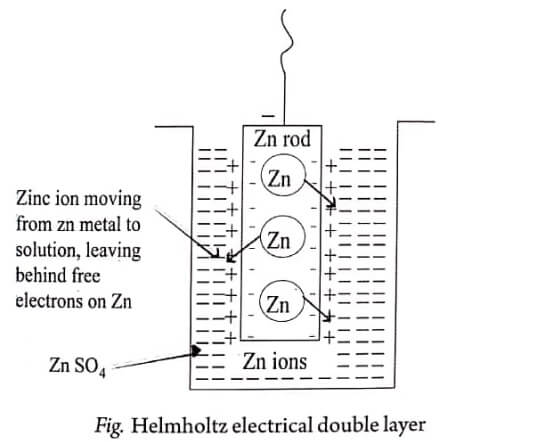
Example 1: Zn in ZnSO4
Consider a zinc rod placed in ZnSO4 solution (Fig). The zinc metal passes into the solution as Zn2+ ions. As the positive Zn2+ ions leave the electrons to the zinc rod, it acquires a negative charge and the solution surrounding zinc rod becomes positively charged.
A dynamic equilibrium is established between the metal atoms and the metal ions and it is represented as follows:
Zn → Zn2+ + 2e-
Due to electrostatic attraction, the metal ions and the electrons orient themselves into an electrical double layer around the metal. This layer is called Helmholtz electrical double layer.
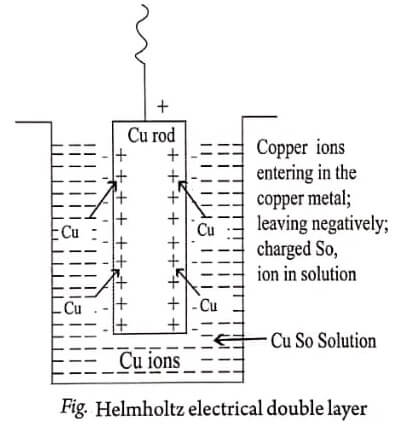
Example 2: Cu in CuSO4
Similarly, when Cu electrode is dipped in CuSO4 solution, Cu2+ ions form the electrolyte deposit over the metal.
Consequently, a potential difference is developed between the electrode, i.e., zinc rod dipped in the ZnSO4 solution or copper rod dipped in the CuSO4 solution. This potential difference is known as electrode potential.
Standard electrons potential (E∘)
The measure of tendency of a metallic electrode to lose or gain electrons when it is in contact with a solution of its own salt of unit molar concentration at 25∘C is called standard electrode potential (E∘).
[sc_fs_faq html=”true” headline=”h2″ img=”” question=”What is Standard Electrode Potential?” img_alt=”” css_class=””] Standard electrode potential is a measure of the tendency of a metal to lose or gain electrons when it is in contact with a solution containing its own ions. It helps us understand which substances are more likely to gain electrons (reduction) and which are more likely to lose electrons (oxidation) in a chemical reaction. [/sc_fs_faq]
| Read More Topics |
| Definition and construction of electrochemical cell |
| How do sodium potassium pumps work |
| Types of softening of water processes |





