To cultivate a specific microbe, following methods are used :
Methods of Culturing Aerobic Bacteria : An atmosphere having oxygen in ample amount facilitates the growth of aerobic or facultative bacteria. For this, a large surface area of medium is used to increase the exposure. Numerous devices, e.g., Fernbach flask, Kolle flask, and Roux bottle are used to provide increased aeration (figure). Oxygen is required for the growth of aerobic bacteria and hence they can easily be grown by incubation in surroundings containing air (i.e., 21% oxygen).
Generally, incubation of medium under normal atmospheric conditions proves satisfactory, when the growth of aerobic or facultative bacteria is desired in tubes or small flasks. However, when the growth of these microbes is desired in huge quantities, increasing the exposure of medium to the atmosphere proves beneficial.
To achieve this, distinctive containers are designed, such that they dispense the medium in shallow layers, thereby facilitating their growth in increased quantities. Constant shaking of inoculated liquid cultures that have been inoculated also increases the exposure to air.
Methods of Culturing Anaerobic Bacteria : There are two categories of methods by which bacteria can be cultivated under anaerobic conditions; methods in which culture media are placed in air-tight containers and oxygen is removed (i-iii), using media in which oxygen diffuses with difficulty (iv), and methods in which a nontoxic reducing agent is added to the culture media (v-vii).
These methods are discussed below :
Boiling : In this technique, the culture media is taken in bottles fitted with a screw cap having a central aperture and a rubber washer. The bottle is then placed in a water bath after loosening the screw cap, and boiled for 10 minutes. Then the bottle is cooled after tightening the screw cap. The microorganism is inoculated using a hypodermic syringe, the needle being plunged through the rubber washer.
Use of Alkaline Pyrogallol : After filling a desiccator with alkaline pyrogallol, culture media are placed in it for oxygen removal. The method can be modified by using a petri dish having a double compartmented lid; one compartment contains sodium hydroxide solution and the other contains pyrogallol solution. The microorganism to be grown is streaked on an agar plate, which is then placed downwards over the lid, the two solutions are gently mixed, and the assembly is incubated.
Mcintosh and Fildes Jar : This jar (made up of strong glass) is fitted with a gas-tight metal lid securely clamped in place. This lid has two terminals for conveying electric current to a coil within the jar; the coil further provides heat to a piece of spongy palladium deposited on asbestos and surrounded with wire gauze.
Inlet and outlet taps are also present in the lid. When the jar is to be used, the culture media are placed within; hydrogen is passed through the taps; current is switched on to heat the spongy palladium; and the oxygen present combines with hydrogen in the presence of the heated catalyst. Care should be taken that the reaction does not become explosive by the external gauze, and thus the jar is placed in a protective box.
Hydrogen is passed through the taps for further 30 minutes; after which the taps are closed and the jar is incubated at suitable temperature. An indicator of anaerobiosis consisting of a mixture of methylene blue and glucose, and made alkaline with sodium hydroxide is added in the jar. This is heated when the alkaline glucose solution turns methylene blue colourless.
This colourless solution is placed in the jar and should remain colourless during incubation; if the blue colour reappears during incubation, the jar is believed to have leakage caused by the atmospheric oxygen. Nowadays, catalysts not requiring prior heating are widely in use.
Growth in Solid and Semi-Solid Media : Under anaerobic conditions, the solid media are poured in a test tube and the microorganisms are inoculated at the bottom using a long wire; this is known as a stab culture. Oxygen is removed by boiling it off when the media are sterilised in the tube.
Diffusion of this oxygen to the bottom of the tube is prevented, owing to the height and nature of the layer medium above. The solid culture media were modified using a nutrient broth in which agar was added in a quantity sufficient to make it semi-solid; this is called sloppy agar.
These media are heated and cooled before inoculation. Anaerobic conditions should be maintained at the container bottom, and the viscosity of the media should be such that diffusion of oxygen is prevented.
Metallic Iron : It is used in the form of wire or small iron nails for producing anaerobiosis. The media are heated for 10 minutes on a boiling water bath. Iron is added to the media after sterilisation by heating for 45 seconds in a bunsen flame. The normal aerobic media used for studying sugar decomposition by aerobes and facultative organisms can be adapted for studying their decomposition by anaerobes.
Cooked Meat : Sufficiently minced and cooked muscle of ox-heart is added to a conventional nutrient broth placed in a 30ml container having a screw cap. The meat should form a 1.5 cm deep layer at the bottom of the liquid media. Muscle tissue contains two reducing systems; in one system reduction occurs by irreversible oxidation of lipids, and other is the reversible parahaematinhaemochromogen system. Robertson created this medium, therefore it is also known as Robertson’s cooked meat medium.
Thioglycollic Acidv : Since 1926 , thioglycollic acid (CH2SHCOOH) is being used as an additive to nutrient broths for cultivating and detecting anaerobes. Gaspak method is used for preparing anaerobic jars (figure). Gaspak is commercially available as a disposable envelope. It contains chemicals generating hydrogen and carbon dioxide on addition of water.
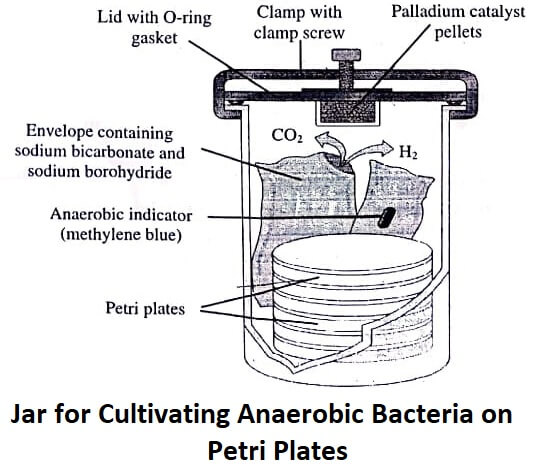
The envelope also contains a cold catalyst which allows hydrogen to combine with oxygen for producing an anaerobic environment. Reduced methylene blue used for verifying the anaerobic condition within the jars remains colourless under anaerobic conditions while turns blue in the presence of oxygen.
| Read More Topics |
| Morphological classification of bacteria |
| Cytoplasm and cytoplasmic inclusions |
| Gram positive and gram negative bacteria |
| Ultrastructure of bacterial cell |





