A material must contain charged particles to be able to conduct electric current. In solids, the current is carried by electrons. Copper, lead, aluminium, iron and carbon are some examples of solid conductors. In liquids and gases, the current is carried by the part of a molecule which has acquired an electric charge, called ions.
These can possess a positive or negative charge, and examples include hydrogen ion H+, copper ion Cu++ and hydroxyl ion OH–. Distilled water contains no ions and is a poor conductor of electricity, whereas salt water contains ions and is a fairly good conductor of electricity.
Electrolysis is the decomposition of a liquid compound by the passage of electric current through it. Practical applications of electrolysis include the electroplating of metals (see below), the refining of copper and the extraction of aluminium from its ore.
An electrolyte is a compound which will undergo electrolysis. Examples include salt water, copper sulphate and sulphuric acid.
The electrodes are the two conductors carrying current to the electrolyte. The positive-connected electrode is called the anode and the negative-connected electrode the cathode.
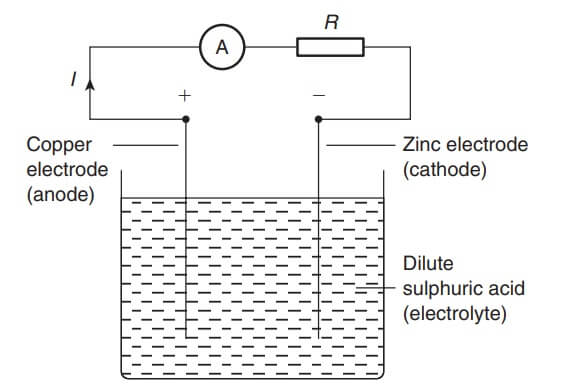
When two copper wires connected to a battery are placed in a beaker containing a salt water solution, current will flow through the solution. Air bubbles appear around the wires as the water is changed into hydrogen and oxygen by electrolysis.
Electroplating uses the principle of electrolysis to apply a thin coat of one metal to another metal. Some practical applications include the tin-plating of steel, silver-plating of nickel alloys and chromium-plating of steel.
If two copper electrodes connected to a battery are placed in a beaker containing copper sulphate as the electrolyte it is found that the cathode (i.e. the electrode connected to the negative terminal of the battery) gains copper whilst the anode loses copper.
| Read More Topics |
| Resistor colour coding and ohmic values |
| Electric current and quantity of electricity |
| Standard symbols for electrical components |
| Electrical / electronic system block diagram |