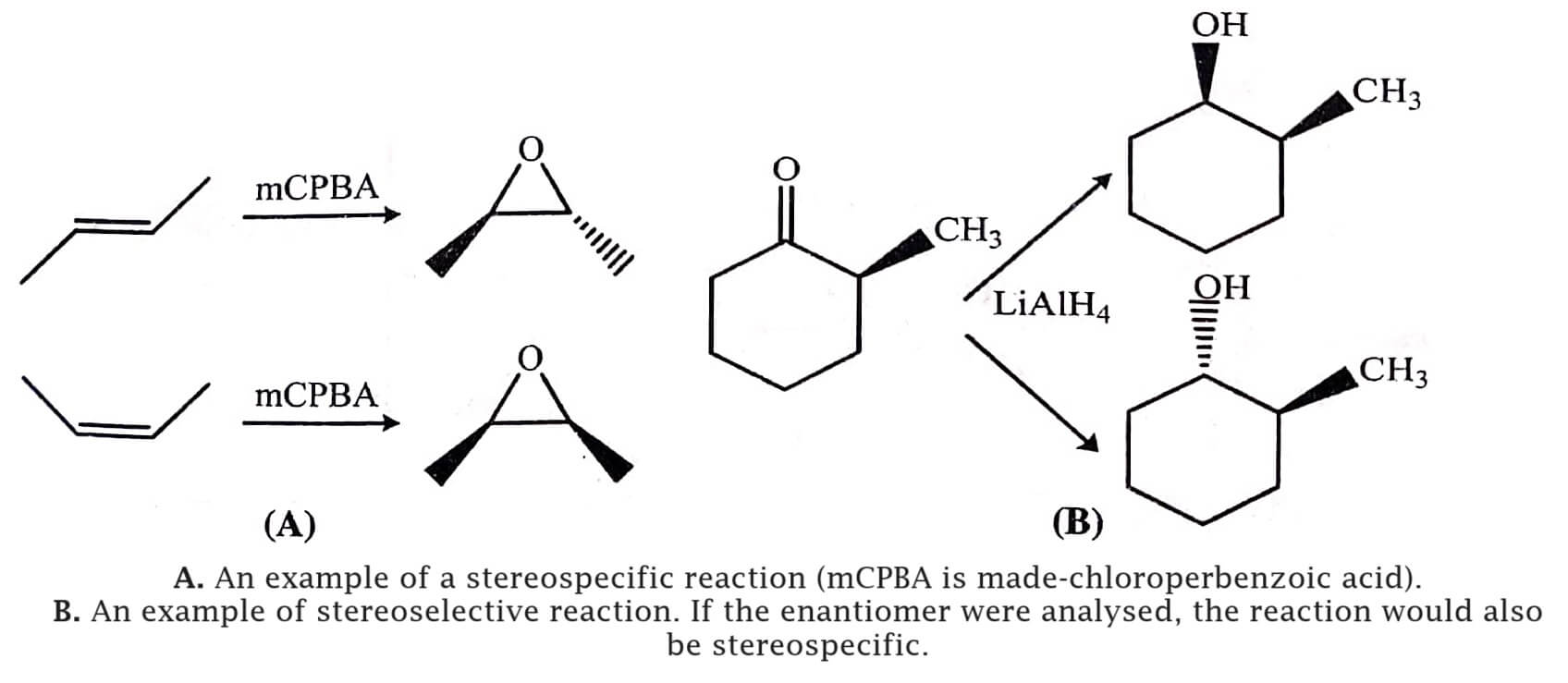
In stereospecific reactions, one stereoisomer of the product is obtained if one stereoisomer of the reactant is present, while different stereoisomers of product are obtained when different stereoisomer of the reactant are present. Thus for determining whether a reaction is stereospecific or not, one has to analyse the product ratio from the different stereoisomer of the reactant. For example, by the epoxidation of 2-butene in the presence of mCPBA, the trans-olefin gives the trans-epoxide and the cis-loefin gives the cis-epoxide (figure).
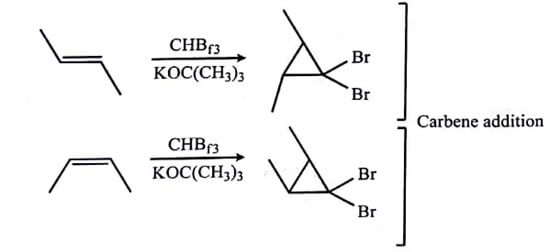
The SN2 reactions are also stereospecific. In these reactions, the these reactions, the inversion of the stereochemistry on stereogenic centres occur consistently, as a result enantiomers of reactants give different enantiomers of the products. Some other examples are given in table (A). If a reaction mixture has 80:20 ratio of stereoisomers, the reaction is called as 80% stereospecific; thus there is no need for a reaction to be perfectly stereospecific.
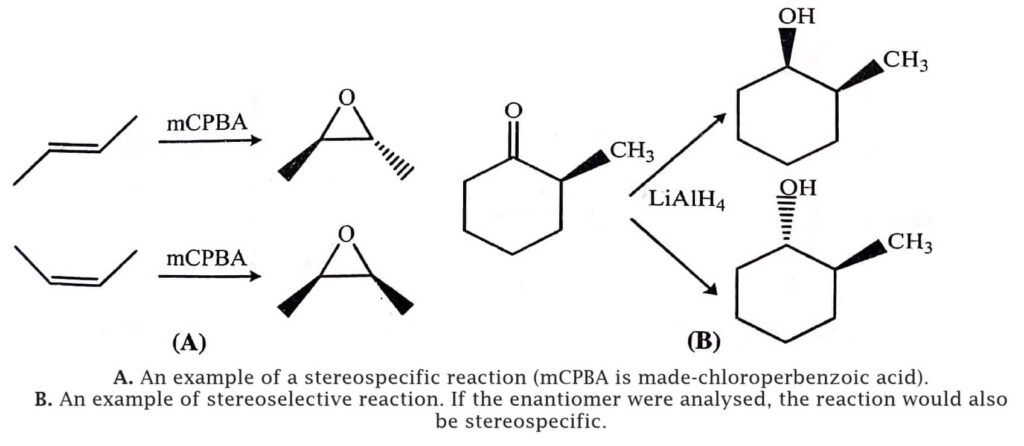
A stereoselective reaction involves a single reactant which gives two or more stereo-isomeric products and even if the preference is very small one or more of these products are chosen over the others. Therefore to determine the stereoselective reactions, only one stereoisomer needs to be analysed. Even sometime the reactant may not exist as stereoisomers, but the reaction can be stereoselective.
The examples are given in table (B).
| Table : Stereospecific Reactions (A), a Stereoselective Reaction (B), and Stereoselective but Not Stereospecific Reaction (C) |
(A) 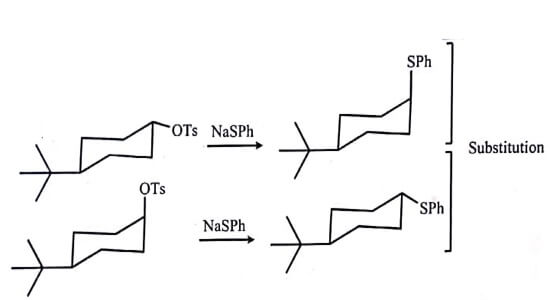 |
 |
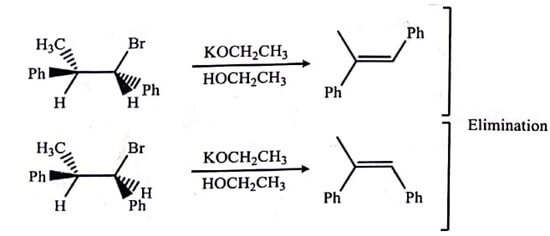 |
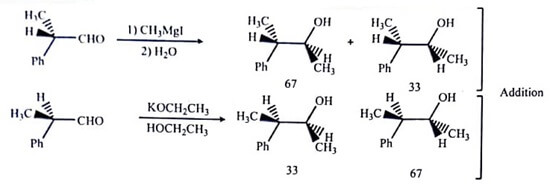 |
(B) 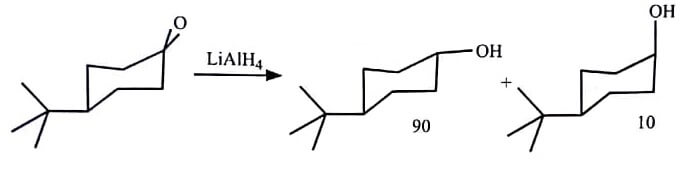 |
(C) 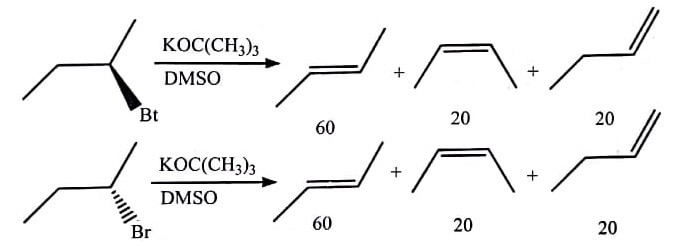 |
If two stereoisomers of the starting material give the same ratio of stereoisomeric products, as long as the ratio is not 50:50, the reaction is said to be stereoselcetive not stereospecific.
For example, it may occur if the mechanisms of reaction for the two stereoisomeric reactants involve a common intermediate which provide two stereoisomeric products with one in excess. Yet, there are also such examples of reactions which involve different stereoisomeric reactants and give the same ratio of stereo isomeric products, even when a common intermediate is not formed as given in table (C). All stereospecific reactions are stereoselective whereas inverse is not true.
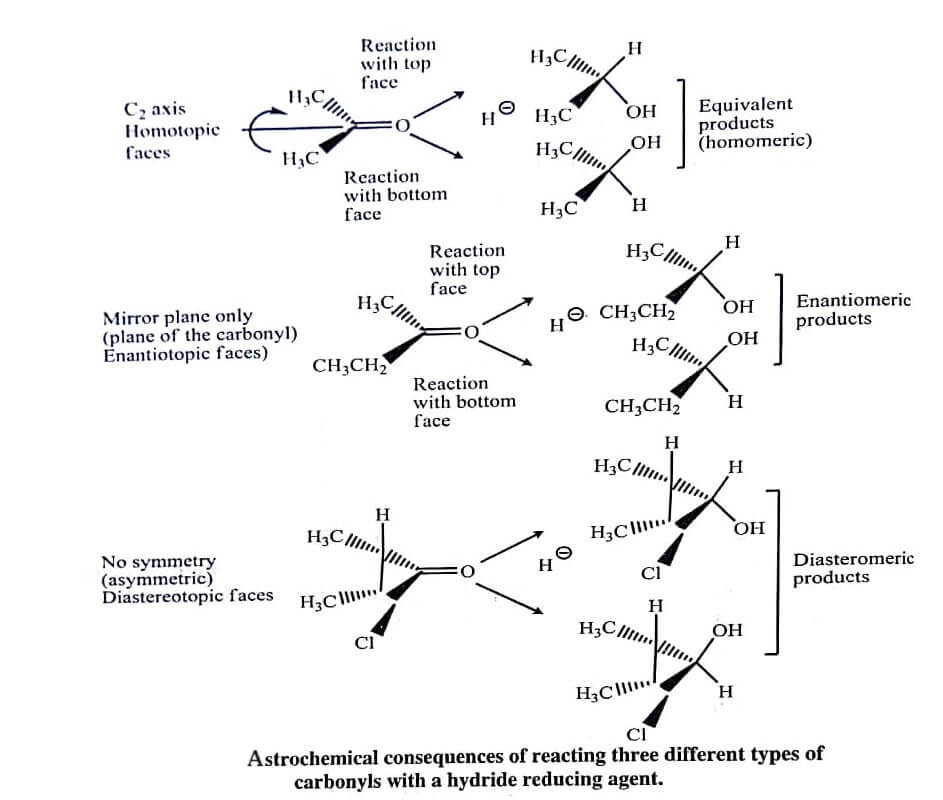
Reduction of (R)-3-chloro-2-butanone is also an example of a stereoselective reaction. In this reaction, the two products are diastereomers therefore this reaction is termed as diastereoselective.
This reaction is also stereospecific, because (S)-3chloro-2-butanone also forms different ratio of products with the same reducing agent. The reaction is known as enantioselective, if one of the two enantiomeric products is formed excessively [as in the reduction of 2-butanone [figure]).
| Read More Topics |
| Nomenclature of geometrical isomers |
| Types of optical isomerism |
| Conformational isomerism |