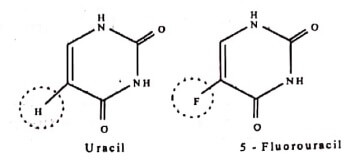
a. Replacement of Fluorine atoms for Hydrogen
b. Replacement of −NH2 group by −CH3
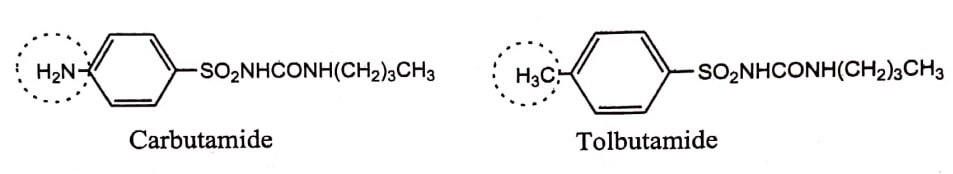
An example of this type is oral hypoglycaemic Carbutamide, the −NH2 group is replaced by −CH3 gives Tolbutamide, which possess extended biological half-lives and reduces toxicity.
c. Replacement of –OH by −SH, NH2
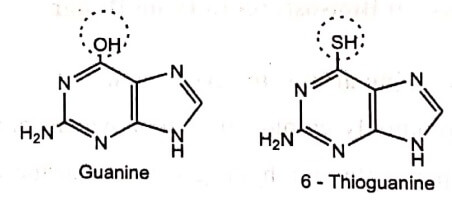
Another example is Calcium channel blockers dihydropyrimidines. Substitution of the hydroxyl with thiol group resulted in enhanced potency.
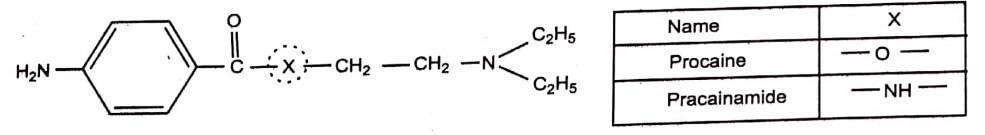
d. Replacement of – O – group by NH group
The replacement of – O – group by NH group in Procaine gives Procainamide. The local anaesthetic activity of Procaine is more than Procainamide due to difference in lipid solubility.
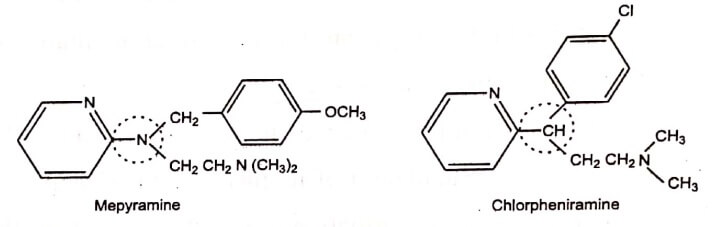
The trivalent substitution of – CH – with – N= is commonly used in modern drug design. Substitution of the pyridinyl amino −N= in Mepyramine by −CH− produces Chlorpheniramine withless sedative effect an undesirable side effect of antihistaminic.
f. Ring replacements
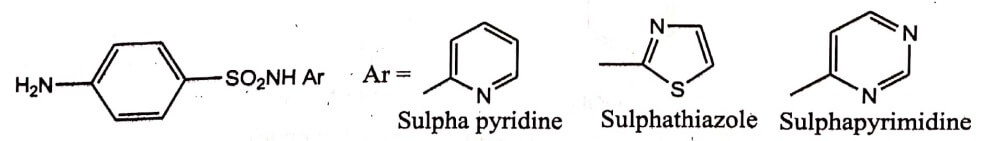
The phenyl group of antibacterial Sulfonamide is replaced by various heterocyclic aromatics which resulted in more active compounds.