Aromatic compounds are organic compounds resembling benzene in their chemical behaviour and possess certain characteristic properties different from those of aliphatic and alicyclic compounds. These characteristic properties are collectively known as aromatic character or aromaticity.
Aromatic compounds possess the following important properties:
- Normally these compounds are cyclic.
- These compounds have high degree of unsaturation still they do not respond to characteristic additional reactions of unsaturated compounds.
- The X-ray and electron diffraction methods show that these compounds are planar aromatic compounds.
- These compounds have high thermodynamic stability indicated by their low combustion and heat of hydrogenation.
- Despite of unsaturation, they undergo electrophilic substitution reactions (such as nitration, halogenation, sulphonation, Friedel-Crafts alkylation and acylation, etc.).
- These molecules are resonance stabilised and the π-electrons in the ring are delocalised.
Following are the requirements to be fulfilled by the aromatic compounds:
- The structure of aromatic compounds should be cyclic with conjugated π-bonds.
- Each atom in the ring should have an un-hybridised p-orbital.
- The un-hybridised p-orbitals should overlap forming a continuous ring of parallel orbitals. For effective overlapping, the structure should be planar (or nearly planar).
- Delocalisation of π-electrons over the ring should result in the lowering of electronic energy.
Valence Bond Theory of Aromaticity
X-ray crystallographic technique illustrates that benzene is a planar, regular hexagon having carbon-carbon bond lengths of 139pm which is intermediate between of the C-C (154pm) and C=C bond length in ethane (134pm); thus benzene exhibit all double bond characters.
This results in unsatisfactory representation of benzene by one Kekule structure. As per the valence bond theory, benzene is a resonance hybrid of two Kekule or canonical forms, each carbon-carbon bond having a bond order of 1.5 .
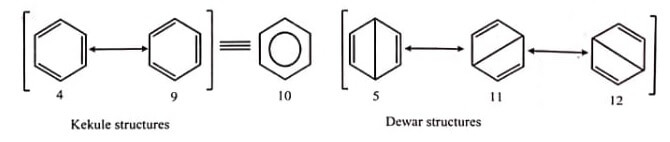
The resonance structures are represented via double-headed arrow. The circle within a benzene ring must represent 6 π-electrons. The three Dewar structures of benzene (5, 11 , and 12) contribute to the resonance hybrid (as per the valence bond theory, approximately 20% in total) and to the extra stability. The Dewar benzene is a bent, nonplanar, and non-aromatic molecule, which gradually returns back to benzene at room temperature.
Although the canonical forms of benzene are imaginary, and benzene is generally represented by one of the Kekule structures. A circle inside a hexagon (figure) symbolises the π-cloud.
| Read More Topics |
| Applications of enzyme immobilization in pharmacy |
| Evaluation of microbial stability of formulations |
| Turbidimetric or tube assay method |