CO2 laser
Carbon dioxide laser is the first molecular gas laser and it was developed by C.K.N. Patel in the year 1963. It is a four level molecular gas laser. In molecular gas lasers, the laser oscillations are achieved by the transition between the vibrational and rotational levels of molecules. Output of these lasers is continuous.
Characteristics of CO2 laser
| Type | – | Molecular gas laser |
| Active medium | – | Mixture of CO2 , N2 and Helium (or) water vapour. |
| Active centre | – | CO2 |
| Pumping method | – | Electric discharge method. |
| Optical resonator | – | Silicon mirrors coated with aluminium. |
| Power output | – | 10kW. |
| Nature of the output | – | Continuous wave and can be pulsed. |
| Wavelength of the output | – | 9.6μm and 10.6 μm |
Principle
The transition between these vibrational and rotational energy levels leads to the construction of molecular gas laser. Here the nitrogen atoms are initially raised to excited state. The Nitrogen atoms delivers the energy CO2 to atoms which has closest energy level to it. Then, transition takes place between the vibrational energy levels of the CO2 atoms and hence laser beam is emitted.
Fundamental modes of vibration of the CO2 molecules
In order to understand the working of this laser one has to recall the rotational and vibrational spectrum of CO2 molecules. The three atoms can be considered as a ring over a straight line, the end atoms being oxygen with a carbon atom C at the centre. There are three modes of vibration.
(i) Symmetric stretching mode
In this mode of vibration the carbon atom is fixed in its position and each oxygen atom can vibrate in opposite direction symmetrical to the carbon atom with each other along a straight line and is known as symmetric mode of vibration.
(ii) Bending mode
In this mode of vibration the oxygen atom and the carbon atom may vibrate at right angles to the line passing through the centre of gravity. This is known as bending mode.
(iii) Asymmetric stretching mode
In this mode of vibration asymmetric mode of operation, the two oxygen atoms may vibrate about the central atom asymmetrically, and at the same time, the carbon atom also vibrate from its mean position.
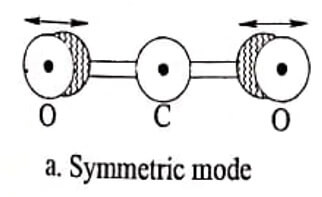
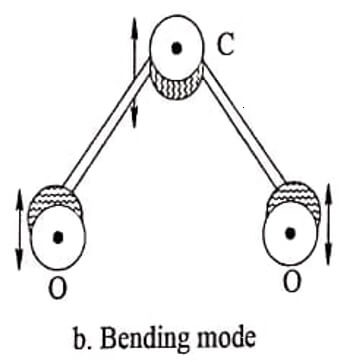
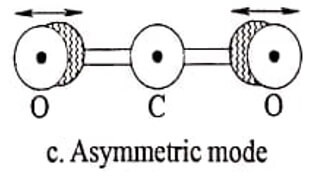 Fig. 1.1 CO2 molecules-different modes of vibrations
Fig. 1.1 CO2 molecules-different modes of vibrations
In addition to these three vibration modes, the molecule can also rotate and therefore, quantized rotational energy levels are also possible. A series of rotational levels are associated with each vibration level.
Construction
It consists of a quartz discharge tube 1m long and 2.5cm in diameter and discharge is produced by d.c. excitation. The tube is filled with a mixture of CO2, N2 and He gases in 1:2:3 proportions respectively.
Along with CO2 there are Nitrogen and Helium gases in the apparatus. The partial pressures of CO2, N2 and Helium around 0.33 torr, 1.2 torr, 7 torr respectively.
Sodium chloride Brewster windows are used at the ends. Two concave silicon mirrors coated with aluminium are provided of which one is completely reflecting and the other is partially reflecting. They form the optical resonator. The output power can be increased by increasing the diameter of the tube.
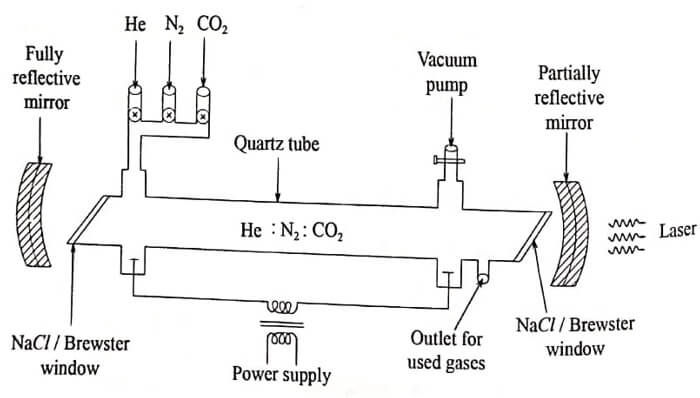
Fig. 1.2 CO2 laser
Working
- The CO2 laser generally uses two additional gases N2 and He. Nitrogen helps to increase the population of atoms in the upper level of CO2 . Helium helps to cool down the discharge tube and also to depopulate the atoms in lower level of CO2 .
- When electrical discharge is passed through the gas, the electrons collide with Nitrogen molecules and are raised to their excited state. (N2 + e*→ N2* + e )
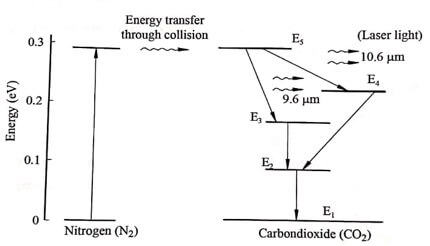
Fig. 1.3 Energy level diagram of laser
- The excited N2 atom undergoes a collision of the second type and makes the CO2 molecules e excited. N2*+CO2→ CO2* +N2
- The frequency of the laser can be pictured in an energy level diagram as shown in (Fig.1.3).
- Since the E5 energy level of CO2 is very close to the excited level of N2 atom, the population of E5 level of CO2 increases rapidly.
- When transition takes place between E5 to E4 laser of wavelength is emitted.
- Similarly, when transition takes place between E5 to E3 laser beam of wavelength 9.6 μm is emitted.
- The operating temperature plays an important role in determining the output power of laser.
- The contamination of carbon monoxide and oxygen will also have some effect on the laser action. To avoid this the unused gases can be pumped out and fresh CO2 must be pumped inside the discharge tube.
- The power output coming from this laser is 10kW. CO2 Laser is also called as Transversely Excited Atmospheric pressure laser (TEA LASER).
Advantages
- The construction of CO2 laser is simple.
- The output of this laser is continuous
- It has extremely high efficiency and very high output power.
- The output power can be increased by extending the length of gas tube.
Disadvantages
- Corrosion may occur in the reflecting plates.
- The contamination of oxygen by carbon monoxide will have some effect on laser action.
Applications
- Because of its high power output, it is used in material processing such as welding, drilling, cutting etc.,
- It is also widely used in open air communication system.
- It is used in laser remote sensing.
- It is used in the treatment of liver, and also in neuro surgery.
| Read More Topics |
| Industrial applications laser welding |
| Medical application of laser |
| Types of semiconductor laser |





