Cholinergic blocking agents are also known as antimuscarinic, anticholinergic, parasympatholytic or cholinolytic drugs. Antimuscarinic drug are those which antagonises the effect of Acetylcholine by binding to muscarinic receptors. Because of the ability to relax smooth muscle, they are also referred as antispasmodics. Nicotinic antagonists which block certain action of Acetylcholine are referred as Ganglionic blockers and neuromuscular blockers.
Structure Activity Relationship
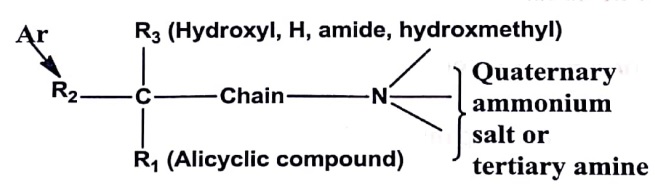
- Nitrogen substituent may be quaternary ammonium salt or a tertiary amine with different alkyl groups.
- Most potent derivatives have Quaternary ammonium salt.
- Tertiary amine protonated at physiological PH also possess antagonist activity.
- Alkyl group can be methyl, ethyl, propyl or isopropyl group.
- The nitrogen atom (cationic site) and pivotal carbon are separated by a chain may be ester, ether or hydrocarbon. Effective binding occurs with anticholinergic agents with ester group since it is similar to acetyl choline.
- R1 or R2 could be aromatic, carbocyclic or heterocyclic, but if both of cyclic it results in more antagonist activity. The rings may be same or different ring. One aromatic moiety is essential for Vander Waals interaction with the receptor in pivotal carbon. One cycloaliphatic or other hydrocarbon (olefenic group) required for hydrophobic interaction with the receptor.
- The R3 group can be hydrogen, hydroxyl, hydroxymethyl, amide or component of the R1 and R2 group. Antagonist with hydroxyl or hydroxymethyl show more potency.
Therapeutic Uses
Anticholinergic drugs of non-quaternary amine derivative will cross the blood brain barrier (BBB) and have therapeutic actions or side effects, involving the central nervous system; if anticholinergic drugs are quaternary amines, they will not cross the blood brain barrier, thus they are devoid of CNS activity.
Therapeutic uses following inhibition of parasympathetic transmission are
- Peripheral
- Central: Antiparkinson and anti-motion sickness.
| Read More Topics |
| Introduction of sympathomimetic agents |
| Stereoisomerism in biphenyl compounds (Atropisomerism) |
| Biosynthesis and catabolism of acetylcholine |