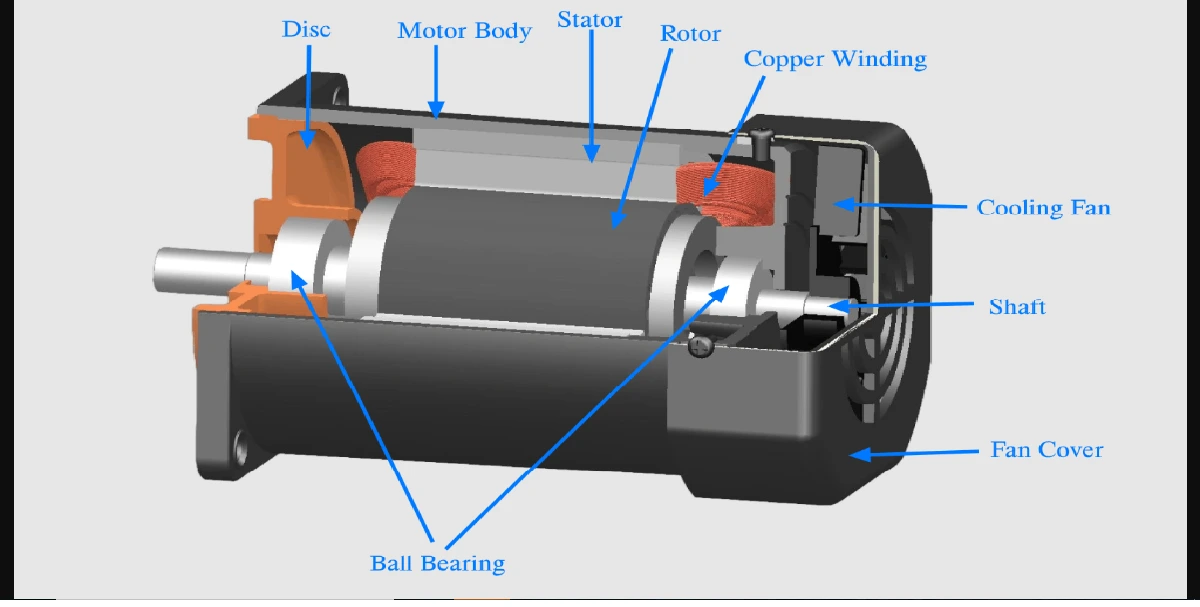
Arsenic, antimony and phosphorus have five valency electrons and when a semiconductor is doped with one of these substances, some impurity atoms are incorporated in the tetrahedral structure. The ‘fifth’ valency electron is not rigidly bonded and is free to conduct, the impurity atom donating a charge carrier.
Indium, aluminium and boron have three valency electrons and when a semiconductor is doped with one of these substances, some of the semiconductor atoms are replaced by impurity atoms. One of the four bonds associated with the semiconductor material is deficient by one electron and this deficiency is called a hole.
![]()
Holes give rise to conduction when a potential difference exists across the semiconductor material due to movement of electrons from one hole to another, as shown in Fig. In this diagram, an electron moves from A to B, giving the appearance that the hole moves from B to A. Then electron C moves to A, giving the appearance that the hole moves to C, and so on.
| Read More Topics |
| Introduction to electromagnetic induction |
| Force on a current carrying conductor |
| Magnetic field due to an electric current |
| Capacitors connected in parallel and series |