Chemistry of Corrosion
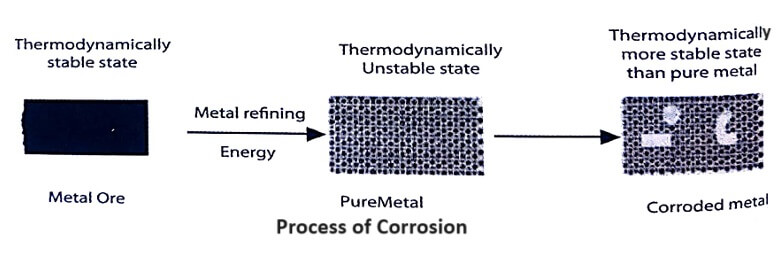
Most of the metals (except noble metals like gold, platinum etc.) exist in nature as ores (metallic oxide, sulphide, carbonate, silicate etc.).
By metallurgical operations, these ores are converted into pure metals. The pure metal will always have higher energy than its ore or metallic compound. The pure metal at higher energy level will always have the tendency to go into the lower-energy state of its compound. This is known as corrosion.
Consequences of corrosion
Corrosion causes the following damages:
- The machines may require frequent replacement.
- The products of corrosion may contaminate other material in the surroundings.
- When one machine fails, the entire plant may have to be stopped thereby resulting in production loss.
- Sometimes over design may be required to increase the life time of the product thus increasing its cost.
- Corrosion may release toxic substances which are hazardous to health.
Effects of corrosion
Effects of corrosion are briefly given below:
- Loss of efficiency of the metal.
- Contamination or loss of the product.
- Increase in maintenance and production costs.
- Plant shut downs due to failure.
[sc_fs_faq html=”true” headline=”h2″ img=”” question=”What is Consequences of Corrosion?” img_alt=”” css_class=””] Corrosion leads to structural weakening and deterioration of materials, compromising integrity and longevity of infrastructure and equipment. It also poses environmental and safety hazards due to potential leaks and failures. [/sc_fs_faq]
| Read More Topics |
| Corrosion in day to day life |
| Electrochemistry – Solved problems |
| Measurement of single electrode potential |






