The approach of solid dispersion for particle size reduction and dissolution and absorption rate enhancement of drugs was first introduced in 1961. The term solid dispersion refers to the dispersion of one or more active ingredients in an inert carrier in a solid state, prepared by the melting (fusion) method, solvent method, or fusion solvent method.
The novel preparation techniques include rapid precipitation by freeze drying, spray drying in the presence of amorphous hydrophilic polymers, supercritical fluid technology, and melt extrusion method. Polyvinyl pyrrolidone, polyethylene glycols, Plasdone-S63020, and surfactants (Tween-80, docusate sodium, Myrj-52, Pluronic-F68, and SLS) are the most commonly used hydrophilic carriers for solid dispersions.
With the approach of solid dispersion using suitable hydrophilic carriers, the solubility of etoposide, glyburide, itraconazole, ampelopsin, valdecoxib, celecoxib, and halofantrine can be improved. The eutectic combination of chloramphenicol/urea and sulphathiazole/urea are the examples for the preparation of a poorly soluble drug in a highly water soluble carrier.
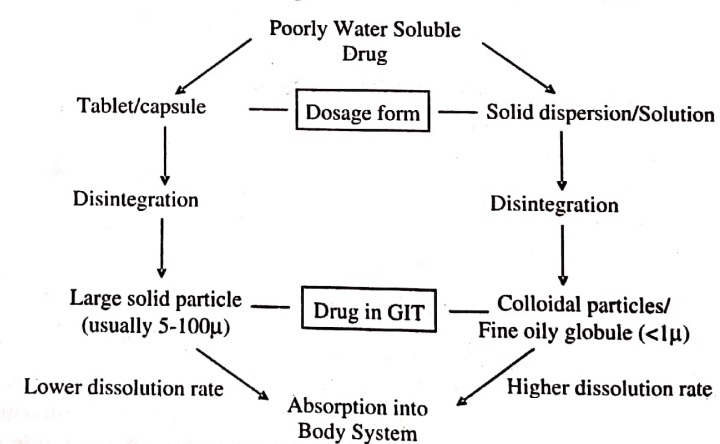
Fig (1) Schematic Representation of Bioavailability Enhancement of a Poorly Water – Soluble Drug by Solid Dispersion
Simple Eutectic Mixture
A eutectic mixture of a sparingly water-soluble drug and a highly water-soluble carrier is thermodynamically regarded as an intimately blended physical mixture of two crystalline components. Increased surface area increases the dissolution rate, thus concluding that the increase in dissolution was due to decreased particle size.
Solid Solutions
Solid solution is a binary system comprising of a solid solute molecularly dispersed in a solid solvent. Solid solutions are also termed molecular dispersions or mixed crystals as the two components crystallise together in a homogeneous one phase system: Reduction of particle size up to the molecular level increases the aqueous solubility of solid solutions, thus they undergo faster dissolution than eutectics and solid dispersions. Solid solutions are prepared by fusion method in which a physical mixture of solute and solvent are melted together and then rapidly solidified. Such systems, prepared by fusion, are termed melts.
- Miscibility between the Drug and the Carrier: Solid solutions are divided into two categories on this basis:
-
- Continuous Solid Solution: In this, the drug and the carrier are miscible in all proportions.
- Discontinuous Solid Solution: In this, solubility of each component in the other is limited.
- Distribution of Drug in Carrier Structure: Solid solutions are divided into two categories on this basis:
-
- Substitutional Crystalline Solid Solution: In this, if the drug and carrier mōlecules are almost of same size, the drug molecule substitutes for the carrier molecules in its crystal lattice.
- Interstitial Crystalline Solid Solution: In this, if the size of drug molecule is 40% or less than the size of carrier molecules, the drug molecules occupy the interstitial spaces in the crystal lattice of carrier molecules (figure 2).
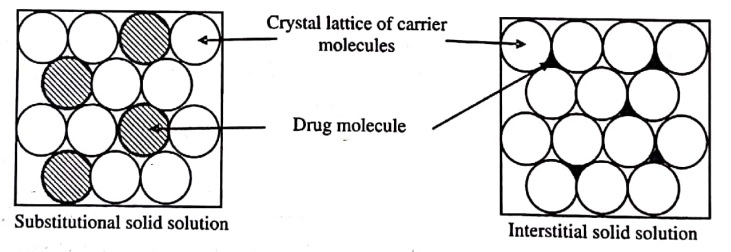
Figure (2): Types of Crystalline Solid Solution
Glass Solution of Suspension
A glass solution is a homogenous system in which a glassy or a vitreous of the carrier solubilises drug molecules in its matrix. PVP dissolved in organic solvents undergoes a transition to a glassy state on solvent evaporation.
Compound or Complex Formation
This system is characterised by complexation of two components in a binary system during solid dispersion preparation. Availability of the drug from the complex depends on the solubility dissociation constant and the intrinsic absorption rate of the complex.
Amorphous Precipitation
When drug precipitates as an amorphous form in the inert carrier, amorphous precipitation occurs. Higher energy state of the drug in this system produces much greater dissolution rates than the corresponding crystalline forms of the drug.
| Read More Topics |
| Factors affecting protein binding |
| Hydrogen bonding and biological action |
| Solubility of the drug |





