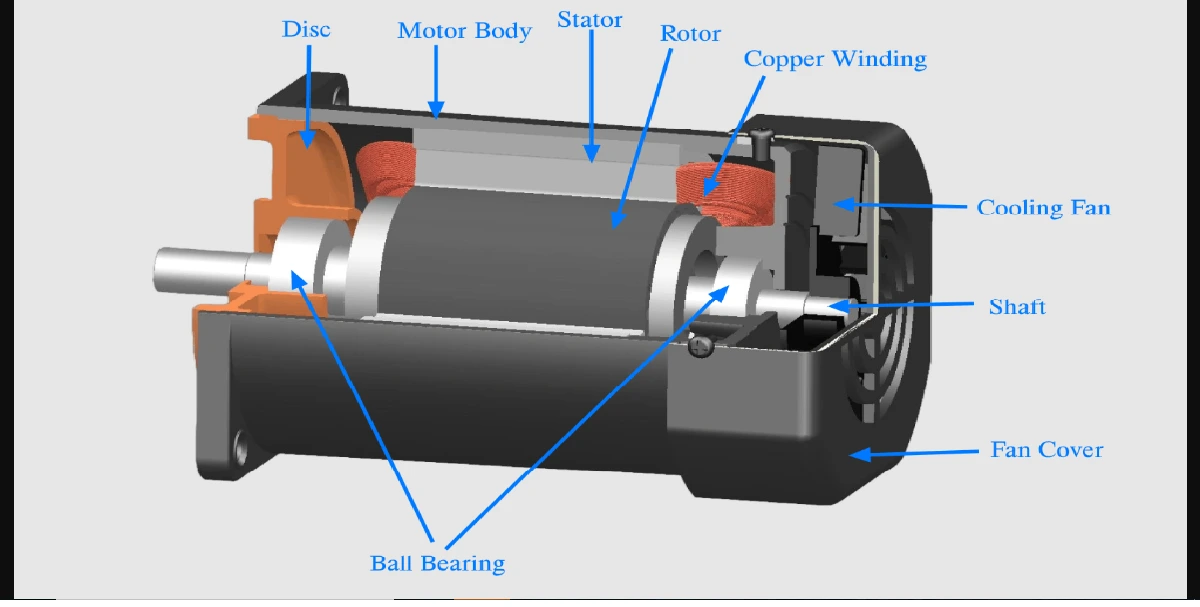
All atoms consist of protons, neutrons and electrons. The protons, which have positive electrical charges, and the neutrons, which have no electrical charge, are contained within the nucleus. Removed from the nucleus are minute negatively charged particles called electrons.
Atoms of different materials differ from one another by having different numbers of protons, neutrons and electrons. An equal number of protons and electrons exist within an atom and it is said to be electrically balanced, as the positive and negative charges cancel each other out. When there are more than two electrons in an atom the electrons are arranged into shells at various distances from the nucleus.
All atoms are bound together by powerful forces of attraction existing between the nucleus and its electrons. Electrons in the outer shell of an atom, however, are attracted to their nucleus less powerfully than are electrons whose shells are nearer the nucleus.
It is possible for an atom to lose an electron; the atom, which is now called an ion, is not now electrically balanced, but is positively charged and is thus able to attract an electron to itself from another atom. Electrons that move from one atom to another are called free electrons and such random motion can continue indefinitely. However, if an electric pressure or voltage is applied across any material there is a tendency for electrons to move in a particular direction.
This movement of free electrons, known as drift, constitutes an electric current flow. Thus current is the rate of movement of charge.
Conductors are materials that contain electrons that are loosely connected to the nucleus and can easily move through the material from one atom to another.
Insulators are materials whose electrons are held firmly to their nucleus.
The unit used to measure the quantity of electrical charge Q is called the coulomb C (where 1 coulomb = 6.24 × 1012 electrons).
If the drift of electrons in a conductor takes place at the rate of one coulomb per second the resulting current is said to be a current of one ampere.
Thus 1 ampere = 1 coulomb per second or
1 A = 1 C/s
Hence 1 coulomb = 1 ampere second or
1 C = 1 As
Generally, if I is the current in amperes and t the time in seconds during which the current flows, then I × t represents the quantity of electrical charge in coulombs,
i.e. quantity of electrical charge transferred,
Q = I × t coulombs
| Read More Topics |
| Primary source of electrical power |
| Electrochemical energy storage |
| Electric energy sources and representations |
| Electric circuit and magnetic circuits |