Setting and hardening of cement
When the cement is mixed with water it produces a plastic mass called “cement paste”. It is due to hydration and hydrolysis reaction that takes place between various constituents of the cement. This results in the formation of gel and crystalline product.
The process of solidification of cement comprises of
(a) Setting (b) Hardening
Setting: It is defined as stiffening of the plastic cement mass into a solid mass due to initial gel formation.
Hardening: It is defined as the development of strength due to the formation of crystallised product.
(I) Initial setting of cement paste is mainly due to the hydration of tricalcium aluminate (C3A). It takes place within 1 day.
3CaO Al2O3 + 6H2O → 3CaO Al2O3 6H2O
Or
C3A + 6H2O → C3A 6H2O
The hydrate tricalcium aluminate combine with gypsum present in the cement to form calcium sulphoaluminate.
C3A + 3CaSO4 2H2O → C3A 3CaSO4 2H2O
(II) After hydration of C3A, tricalcium silicate (C3S) begins to hydrate to give tobermonite gel and crystallization of calcium hydroxide. It takes 7 days.
2(3CaO SiO2) + 6H2O → 3CaO SiO2 3H2O + 3Ca(OH)2
or
2C3S + 6H2O → C3S2 3H2O + 3Ca(OH)2
(III) Dicalcium silicate (C3S) also gets hydrolysed to give tobermonite gel and crystallization of calcium hydroxide. It gets hydrated in 7- 28 days.
2(2CaO SiO2) + 4H2O → 3CaO 2SiO2.3H2O +Ca(OH)2
or
2C2S + 4H2O → C3S2 3H2O +Ca(OH)2
(IV) The hydration of tetracalcium aluminoferrite also takes place very slowly.
4CaO Al2O3 Fe2O3 + 7H2O → 3CaO Al2O3 6H2O + CaO Fe2O3 H2O
or
C4AF + 7H2O → C3A 6H2O + CF H2O
Sequence of changes during setting and hardening
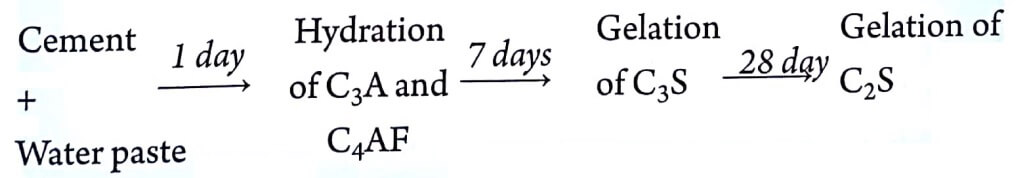
| Read More Topics |
| How are the cement classified? |
| Describe the manufacture of Portland cement |
| Important aspects of cement |





