Conformational isomers or rotamers are infinite numbers of momentary arrangements of groups or atoms in space that results due to the rotation about a single bond. Factors affecting conformational stability of a molecule are:
Steric Strain: Steric strain or Van der Waal’s strain is developed when distance between two non-bonded atoms is less than the sum of their Van der Waal’s radius.
Torsional Strain: It is a repulsive interaction between bond pairs of both carbons which affect stability of conformation. Lower the torsional strain greater will be the stability of conformation.
Dipole-Dipole Interactions: Lower the distance between like poles lower will be the stability similarly greater the distance between like poles greater will be the stability of the conformations.
Angle Strain: Any deviation from normal bond angle develops angular strain. Lesser the angular strain greater will be the stability of conformation. Angular strain develops only in cyclic compounds.
Intramolecular Hydrogen: Intramolecular hydrogen bonding provides stability to the conformers. It stabilises gauche form more therefore it becomes more stable when compared to the antiform. For example, conformations of ethane.
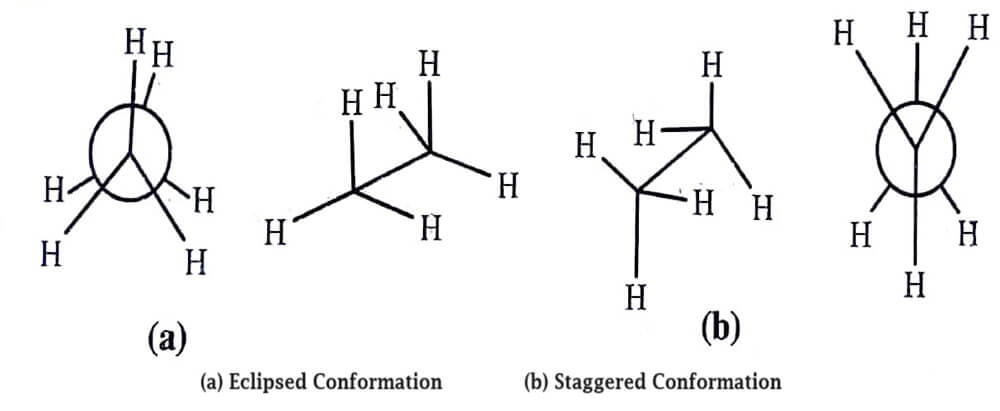
Ethane has infinite number of conformations but only two extreme conformations.
staggered and eclipsed are most important:
Generally, the order of stability of various conformations is anti > gauche > partially eclipsed > fully eclipsed.
If intramolecular hydrogen bonding is present then the order of stability is gauche > anti > partially eclipsed > fully eclipsed.
| Read More Topics |
| Stereospecific and stereoselective reactions |
| Nomenclature of geometrical isomers |
| Types of optical isomerism |





