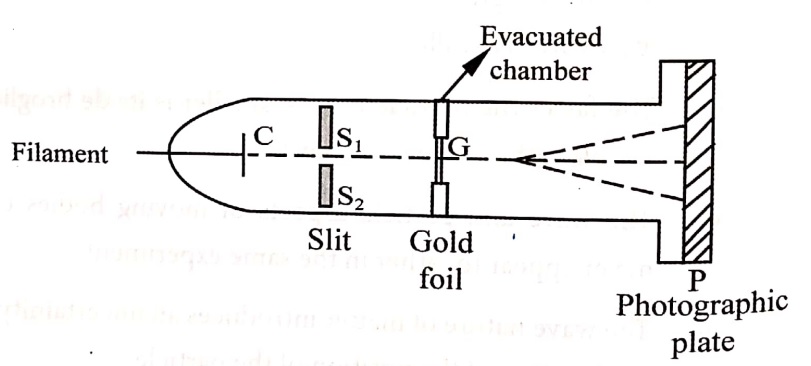
The de-Broglie hypothesis is confirmed by the experiment conducted by G.P. Thomson in 1927. This G.P. Thomson experiment is the evidence for the electrons to behave as waves.
G.P. Thomson performed experiments in which a narrow beam of electrons are accelerated through a narrow hole and made to incident on a thin gold foil by the application of high voltage from 10,000 to 50,000 volts. In this experiment, the diffraction pattern is obtained by the electrons in the gold foil. This diffraction pattern are considered very much similar to X-ray diffraction pattern. The diffraction pattern is obtained by only when wave is associated with particle. Hence, G.P. Thomson explains the concept of matter waves.
Experimental setup and Working
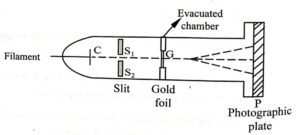 Fig. (1) G.P. Thomson Experiment
Fig. (1) G.P. Thomson Experiment
- This experiment consists of a discharge tube.
- The complete apparatus is kept in a high vacuum chamber so that the electrons may not lose their energy colliding with molecules of air or any inside the tube.
- The electrons are emitted from the filament and only some accelerated electrons are passing through cathode ‘C’.
- Next these electrons are passed through two slits S1 and S2 and a thin pencil beam of electrons is obtained.
- This electron beam is allowed to fall on a thin foil ‘ G’ of gold or aluminium of order 10−6 cm.
- The photograph of electron beam from the foil is recorded on the photographic plate ‘ P ‘.
- Hence, a pattern consists of concentric rings.
- The diffracted electrons produce the diffraction rings as shown in (Fig.2).
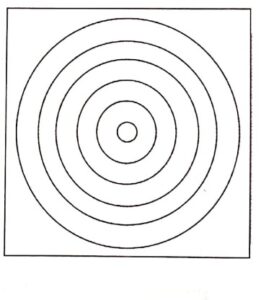 Fig. (2) Diffraction pattern
Fig. (2) Diffraction pattern conclusion
To conclude, this pattern is due to the electrons and not due to the X-rays. The cathode rays inside the tube are affected by the magnetic fields. The beam shifting considerably along the field is observed. Hence, we can conclude that the pattern obtained is due to electrons only since X-rays are not affected by electric and magnetic fields.
| Read More Topics |
| Linear flow of heat along a bar |
| Characteristics of De-Broglie waves |
| Verification of newtons law of cooling |