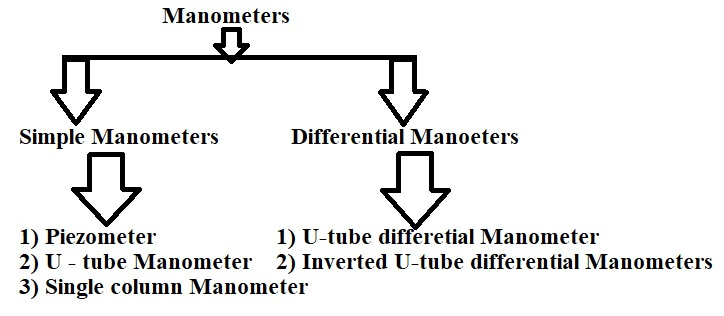
Manometers is the device which is used for measuring the pressure at a point in a fluid by balancing the column of fluid by the same or another column of liquid.
Simple Manometers
Simple manometers are used to measure the pressure at a point in a fluid contained in a pipe or a vessel. It consists of a glass tube having one of its end connected to a point where the pressure is to be measured and other end remains open to atmosphere.
Piezometer
A piezometer is the simplest form of manometers which can be used for measuring moderate pressure of liquids. It consists of a glass tube in which one end is connected to the wall of the pipe or a vessel and the other end is open to the atmosphere as shown in figure.
The pressure at any point in the liquid is indicated by the height of the liquid in the tube above that point which can be read on the scale attached to it. If at a point A, the height of the liquid is ‘h’ in piezometer tube, then pressure at A is given by p=w×h

where
w = Specific weight of the liquid.
Piezometers measure gauge pressure only since the surface of the liquid in the tube is subjected to atmospheric pressure. Piezometers are not suitable to measure vacuum pressures.
U -tube Manometer
Piezometers cannot be employed when large pressures in the lighter liquids are to be measured, since it would require very long tubes which cannot be handled conveniently. Furthermore, gas pressures cannot be measured by Piezometers because a gas forms no free atmospheric surface. These limitations can be overcome by the use of U-tube manometers.
A U-tube manometer consists of a glass tube bend in U-shape, one end of which is connected to a point at where the pressure is to be measured and the other end remains open to the atmosphere as shown in Figure. U-tube contains a liquid of specific gravity greater than that of the fluid of which the pressure is to be measured.

For gauge pressure : Let A be the point at which the pressure is to be measured. X-X is the datum line.
Let h1 ⇒ height of the light liquid above the datum in the left limb.
h2 ⇒ height of heavy liquid above the datum in the right limb.
S1 ⇒ Specific gravity of light liquid.
S2 ⇒ Specific gravity of heavy liquid.
When the pressure in the tube is same as atmospheric pressure, the pressure above the horizontal datum line X-X in the left column should be same as in the right column.
Pressure above X-X in the left column = h+h1S1
Pressure above X-X in the right column = h2S2
Equating these two pressures,
h+h1S1=h2S2
h=h2S2-h1S1
For vacuum pressure
Pressure above X-X in the left column = h+h1S1+h2S2
Pressure above X-X in the left column = 0
h=-(h1S1+h2S2)
| Read More Topics |
| Zeroth law of thermodynamics |
| Optical pyrometer working |
| Capsule pressure sensor |