If the blood flows rapidly and uniformly to the entire body tissues, differences in the degree of distribution between tissues will indicate the differences in the tissue penetrability of the drug. Thıs process will be tissue permeability rate-limited. In distribution of drugs, following are the two main rate-determining steps:
1)Rate of Tissue Permeability: Tissue permeability of a drug mainly depends on two factors, i.e., the physicochemical properties of the drug and the physiological barriers restricting diffusion of drug into tissues. Molecular size, degree of ionisation, and partition coefficient are the main physicochemical properties influencing drug distribution.
Most of the drugs having molecular weight less than 500-600 Daltons can feasibly diffuse into the extracellular interstitial fluids by crossing the capillary membrane. But, penetration of drugs from the extracellular fluid into the cells is determined by the molecular size, ionisation constant, and drug lipophilicity. Only small, water-soluble molecules and ions of size less than 50 Daltons enter the cell via aqueous filled channels, while the larger sized particles can be passed by a specialised transport system.
The tissue permeability of a drug is mainly determined by its degree of ionisation. The ionisation and diffusion of drugs into cells decide the pH of blood and extravascular fluid. A drug which remains unionised at these pH values permeates the cells with a faster rate.
The pH of blood and ECF generally remain constant at pH 7.4 , therefore they do not affect drug diffusion unless conditions like systemic acidosis or alkalosis are static or remain unaltered.
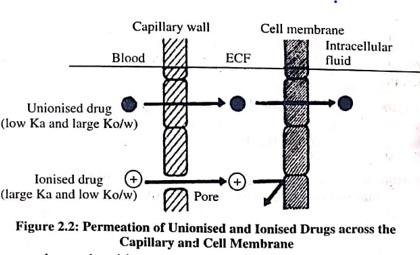
Drugs are mostly weak acids or weak bases, and their degree of ionisation at plasma or ECF pH depends on their pKa value. All polar and hydrophilic drugs get ionised at plasma pH, cannot penetrate the lipoidal cell membrane, and tissue permeability is the rate-limiting step in their distribution. Only lipophilic unionised drugs rapidly cross the cell membrane.
Table 1: Distribution of Acidic Drugs in CSF
| Drugs | Relative Acidity | Effective Ko/w at pH 7.4 | Relative Rate of Distribution |
| Thiopental | Weaker acid | 2.0 | 80 |
| Salicylic acid | Stronger acid | 0.0005 | 1 |
Permeability is the rate-limiting step in drug distribution:
i) If the drug under consideration is ionic, polar, or water-soluble.
ii) If the highly selective physiological barriers restrict such drugs to diffuse into the cell.
On the other hand, perfusion is the rate-limiting step in drug distribution:
i) If the drug is highly lipophilic.
ii) If the membrane across which the drug is to diffuse is highly permeable (such as those of the capillaries and muscles).
Only highly lipophilic drugs (like thiopental) can cross the most selective barriers such as the blood-brain barrier (BBB), while highly permeable capillary wall allows almost all the drugs (except those bound to plasma proteins) to pass. In both the cases, the rate of blood flow or perfusion to the tissue is the rate-limiting step. Thus, greater the blood flow, faster the distribution.
2)Rate of Blood Perfusion: Perfusion rate is the volume of blood that flows per unit time per unit volume of the tissue: Its unit is ml/min/ml of the tissue.
If represents tissue/blood partition coefficient of drug, the first-order distribution rate constant (Kt) is expressed as:
eqn (2)
The tissue distribution half-life is given as:
eqn (3)
![]()
The extent up to which a drug is distributed in a particular tissue or organ depends on the size of tissue (i.e., tissue volume) and the tissue/blood partition coefficient of the drug. This can be explained by taking the example of thiopental (a lipophilic drug), which has a high tissue/blood partition coefficient towards the brain and higher for adipose tissue.
Because brain (site of action) is a highly perfused organ, thiopental diffuses very rapidly into the brain and shows a rapid onset of action when given intravenously. Adipose tissues are poorly perfused, therefore, distribution occurs very slowly with the same drug. If thiopental concentration in the adipose tissue shifts towards equilibrium, the drug rapidly diffuses out of the brain and localises in the adipose tissue whose volume is 5 times more than brain and has greater affinity for the drug. Such tissue redistribution results in rapid termination of action of thiopental.
| Read More Topics |
| Absorption of drugs from non-per oral extra vascular routes |
| Introduction to medicinal chemistry |
| Nomenclature of geometrical isomers |





