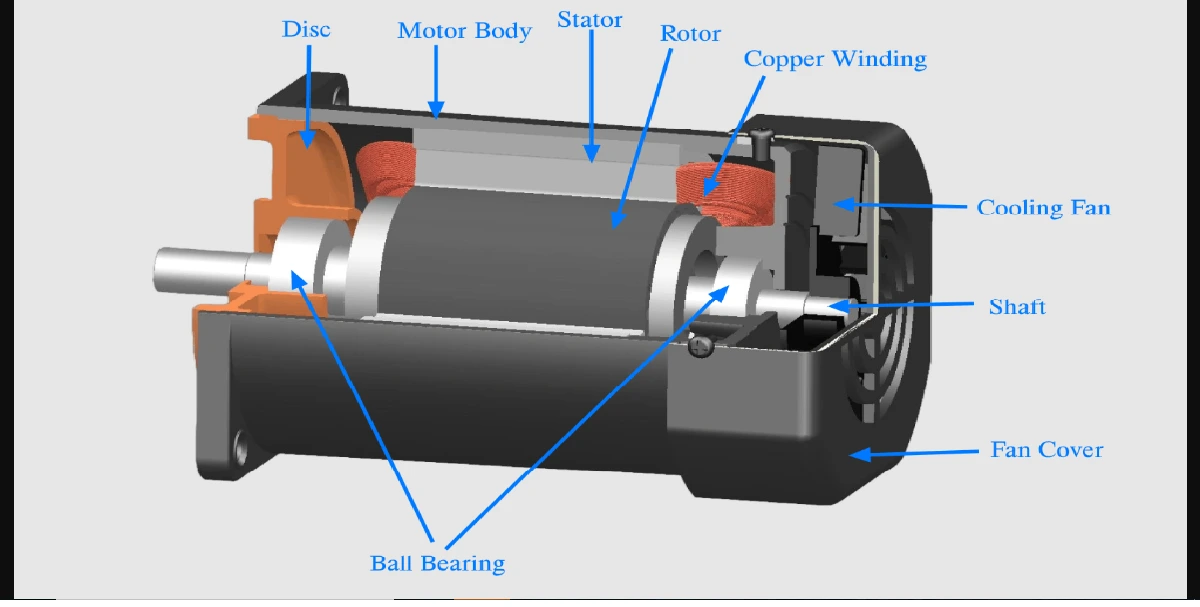
Silicon, an element commonly found on sandy beaches and in window glasses, is the substrate of choice for building integrated circuits.
Silicon belongs to a class of materials called semiconductors. which offers remarkable electrical characteristics and controllability for electronics circuits.
Semiconductor silicon is the most common material in the MEMS field. Naturally, the electrical and mechanical properties of silicon are of great interest.
A semiconductor is fundamentally different from conductors (metals) and insulators (glass or rubber). As its name suggest, a semiconductor is a material whose conductivity lies between that of a perfect insulator and a perfect conductor.
However, this only tells half of the story about why semiconductors are used heavily for modern electronics. More importantly, the conductivity of a semiconductor material can be controlled by a variety of means, such as intentionally introduced impurities, externally applied electric field, charge injection, ambient light, and temperature variations.
These control “levers” lead to many uses of the semiconductor materials, including bipolar junction transistors, field effect transistors, solar cells, diodes, and sensors for temperature, force, and concentrations of chemical species (chemical field effect transistors, or ChemFET).
Certainly, the value of conductivity of a semiconductor piece is of tremendous interest. The macroscopic resistance and conductance are related to the microscopic conductivity.
One of the most basic exercises in MEMS is to find the conductivity value of a semiconductor silicon piece based on its doping concentrations. This constitutes the major focus of this section.
assume a reader is familiar with basic knowledge of charge, voltage, electric field, current, and the Ohm’s law.
Semiconductor Materials
The unique electrical properties of semiconductors stem from their atomic structures. In this section, let us look at how conductivity of semiconductor silicon is originated.
Silicon is a Group IV element in the periodic table. Each silicon atom has four electrons in its outermost orbit. As a consequence, each silicon atom in the crystal lattice shares four covalent bonds with four neighboring atoms.
Silicon atoms reside in a crystal lattice. The inter-atomic spacing between atoms is determined by the balance of atomic attraction and repulsive forces. The density of silicon atoms in a solid is 5×1022 atoms/cm³ at 300 K.
Electrons that covalently bond to the orbits of silicon atoms cannot conduct current and do not contribute to bulk conductivity. The conductivity of a semiconductor material is only related to the concentration of electrons that can freely move in the bulk.
An electron bond toa silicon atom must be excited with enough energy to escape the outermost orbit of the atom for it to participate in bulk current conduction.
The statistically minimal energy needed to excite a covalently bonded electron to become a free charge carrier is called the bandgap of the semiconductor material.
It corresponds to the energy necessary to break a single covalent bond between two atoms. The bandgap of silicon at room temperature is approximately 1.11 eV, or 1.776 × 10-19 J. For more detailed information, refer to classic textbooks on solid-state electronics devices ([1,2]).
Electrons can receive energy and be liberated from its host atoms by a number of means, including lattice vibration (e.g., temperature rise) and absorption of electromagnetic radiation (e.g., light absorption).
Temperature plays an important role in determining the concentration of free electrons in a bulk. In fact, semiconductor silicon is insulating at absolute zero temperature (0 K), when no free-electrons are available to conduct current.
Higher temperatures lead to greater concentrations of free charge carriers and better conductivity.
A metal conductor, in contrast, consists of metal atoms that are linked to one another with metallic bonds, which are generally weaker than covalent bonds. Electrons can readily break free and participate in current conduction. The equivalent bandgap associated with metals is zero.
Hence the conductivity of a metal conductor is always high and not sensitive to changes of temperature and light conditions.
An insulator, on the other hand, involves atomic bonding that is much stronger than that of a semiconductor material, such as ionic bonds. In other words, the bandgap of insulators is much greater compared with that of semiconductors.
Since it is difficult for electrons to break loose from the atom orbits, the concentration of free charge carriers and the conductivity of insulator materials are very low.
Silicon is not the only semiconductor material used for MEMS. Other semiconductor materials, such as germanium, polycrystalline germanium, silicon germanium, gallium arsenide (GaAs), gallium nitride (GaN), and silicon carbide (SiC), have also been used.
The bandgaps of these materials are different from that of silicon [3,4].
Certain organic materials also exhibit semiconductor characteristics. Organic semiconductors are being investigated for flexible circuits and displays. These generally involve different device architecture and fabrication methods compared with inorganic semiconductors. This topic is beyond the scope of this text; however, interested readers can read the following referenced papers to get acquainted on this emerging research topic [5-8].
| Read More Topics |
| Types of electrical breaking |
| Primary source of electric power |
| Reactive ion etching |
| Types of solar collectors |