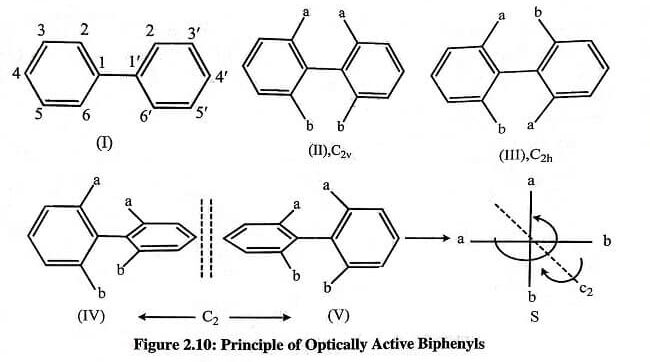
In biphenyl (I) two phenyl groups are linked by a single bond (sp2 -sp2), known as pivotal bond. The distance between ortho H‘s in adjacent rings in the planar conformation is significantly greater (0.29nm) than twice the Van der Waals radius of hydrogen (2 x 0.12nm). Due to this, the rotation around the pivotal bond is not hampered by steric factors.
A dissymmetrically substituted phenyl group (does not posses a vertical plane of symmetry) is two-dimensionally chiral and a planar combination of two such groups would give two (cis and trans) diastereomers (II) and (III). Whereas, a non-planar combination would give two enantiomers (IV) and (V) of C2 symmetry, and the arrangement of abab corresponds to the general chiral structure. These molecules are axially chiral with the line joining C-1 and C-1′ as the chiral axis.
Hence, it is clear that in order to have the biphenyls to be enantiomeric (resolvable) an additional condition is required, i.e., the planes of the two aryl group must be kept non-coincident. It can be achieved by introducing bulky group at the ortho positions due to which the planar conformation get destabilised by steric repulsion.
An approximate energy diagram is shown (Figure 2.11) for a 360° (θ) rotation around the pivotal bond. The inter-ring resonance is lowest at 90° therefore the true situation may be that of a double minimum, when θ is around 90° and 270°, as shown in the figure 2.11. Thus the preferred conformations of the enantiomers are those in which the two phenyl planes are approximate but are not exactly perpendicular to each other.
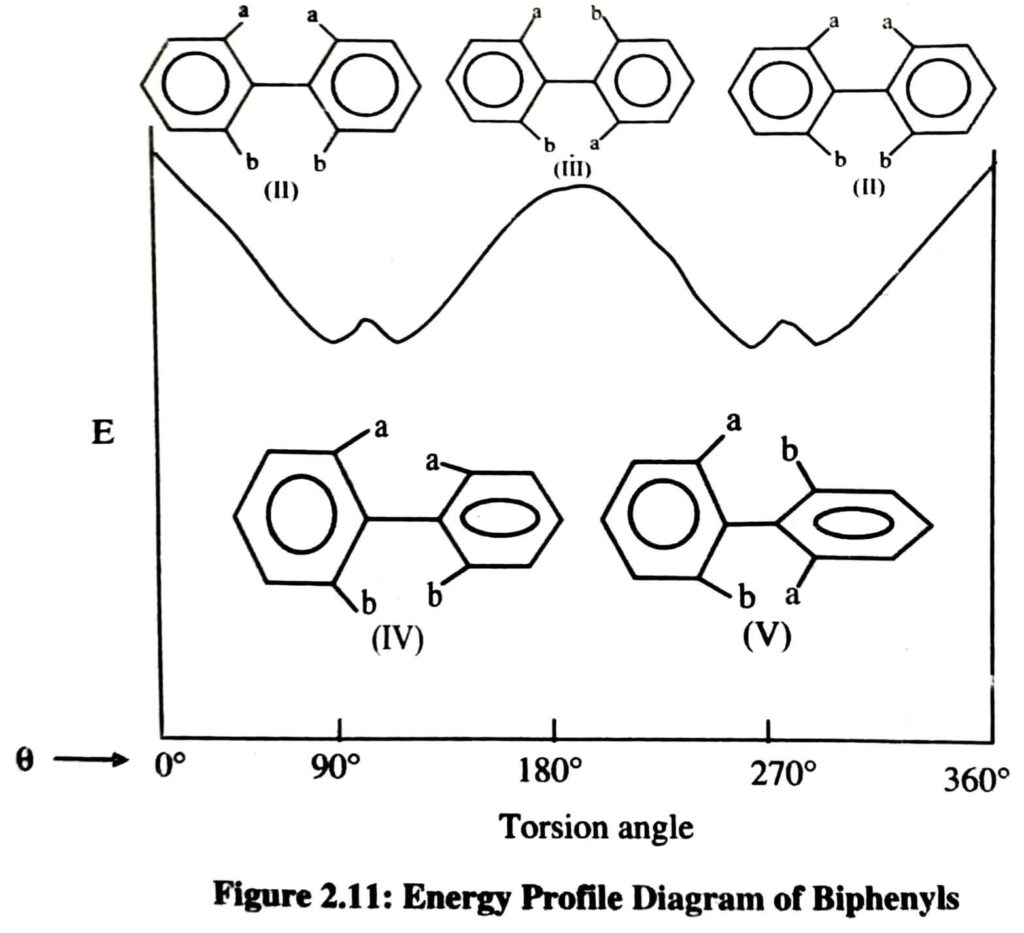
It is also observed that the two diastereomeric planar conformations (II) and (III) represent the energy maxima, i.e., one with same groups on the same side (cisoid) with higher energy and the other with same groups on opposite sides (transoid). Therefore, racemisation occurs with greater case by the transoid configuration. Thus the bulkier the ortho substituents, the higher will be the energy barrier separating the enantiomers. When this energy barrier exceeds 80-100 KJ mol‾1, the stereoismers can be separated at room temperature. Such isomerism which results due to restricted rotation around a single bond is termed as atropisomerism and the isomers are known as atropisomers. Actually, they are torsional isomers around single bonds.
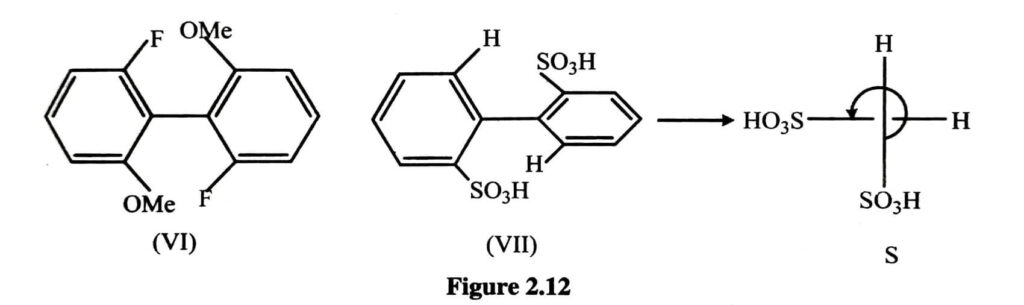
Due to the higher bulk of SO3H the two ortho substituents is resolvable as given in figure2.12.
Along with physical proofs like X-rat diffraction, dipole moment measurement, electronic spectroscopy (UV) NMR, etc., the direct chemical indication for the non-planar configuration of the optically active biphenyl can be provided by the example involving the compound (VIII) and compound (IX) (figure 2.13) which are resolvable. However, the compound (X) in which the ortho positions are combined through planar rings is non-resolvable due to the non-bonded interaction has been changed into a bonded one.

| Read More Topics |
| Physiological barriers for drug distribution |
| Tissue permeability of drugs |
| Enzymes involved in drug metabolism |





