Chelation/Complexation
Complexes or co-ordination results from a donor-acceptor mechanism (donating accepting electron or rather an electron pair) or Lewis acid-base reaction (donating accepting protons). Since complex drug cannot cross the natural membranous barriers, they reduce the rate of absorption of the drug. The compounds that are obtained by donating electrons to metal ion with the formation of a ring structure are called chelates. The compounds capable of forming a ring structure with a metal atom are termed as ligands.
Importance of Chelates in Medicine
(a) Antidote for metal poisoning
(i) Dimercaprol is a chelating agent. It is an effective antidote for organic arsenical and also used to treat poisoning by Gold and Arsenic.

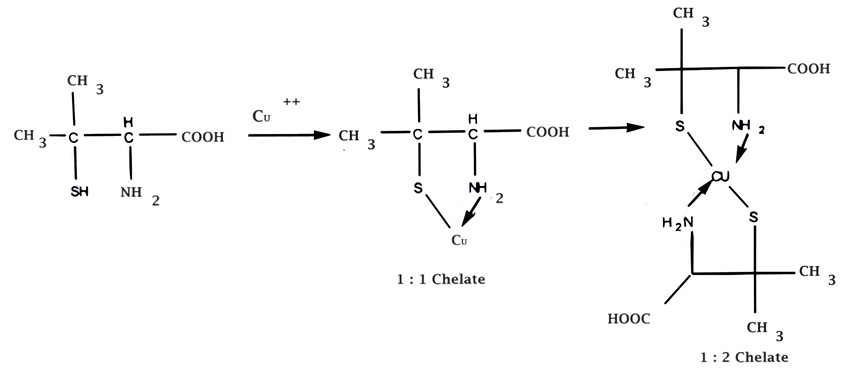
(ii) Penicillamine used in Wilson’s disease because it forms water soluble chelates with copper.
(b) Removal of metals causing deterioration in pharmaceuticals
Water-soluble ligands are referred as sequestering agents. Certain drug, eg. Epinephrine and ascorbic acid are destroyed by oxidation and metals catalyze this oxidation. Common sequestering agents EDTA is used which forms a water-soluble ligand.
(c) Undesirable side effects caused by drugs, which chelates with metals
A side effect of Hydralazine an antihypertensive agent is formation of anemia and this is due to chelation of the drug with iron.
(d) 8-Hydroxy quinoline and its analogs act as antibacterial and antifungal agent by complexing with iron or copper.
| Read More Topics |
| Factors affecting conformational stability |
| Classification of heterocyclic compounds |
| Tissue permeability of drugs |





