For the evaluation of efficiency, potency, and reactivity of disinfectants following techniques are used:
- Tube dilution and agar plate method,
- Cup-plate, filter paper, and cylinder plate method,
- Ditch-plate or giant colony method,
- Kelsey-Sykes test,
- In-use dilution test,
- Phenol coefficient test – Rideal-Walker test, and
- Chick-Martin test.
Tube Dilution and Agar Plate Method
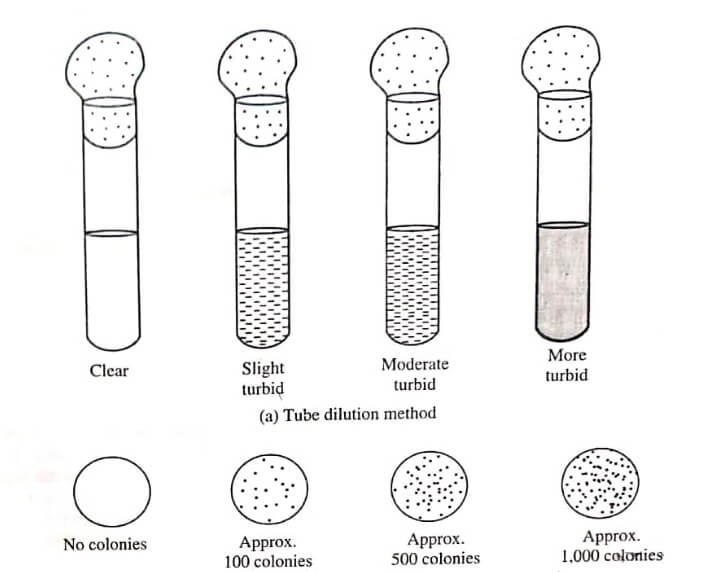
The chemical disinfecting agent is introduced into the agar medium or nutrient broth, medium. After that the medium are inoculated with the test microorganisms. The test tubes containing medium and microorganism is incubated at 30−35°C for 2−3 days and then the results are observed in the form of turbidity or colonies. These turbid colonies are recorded and the activity of given disinfectant is compared as shown in the figure.
Cup-Plate, Filter Paper, and Cylinder Plate Method
In this method, heating is performed for melting the agar, and cooled up to 45°C. The agar medium is inoculated with the test microorganisms and poured into a sterile petri dish. In the cup-plate method, when the inoculated agar has been solidified, holes of about 9 mm in diameter are made by cutting the medium with sterile cork borer and the antimicrobial agents are directly placed in these holes (figure).
In the filter paper and cylinder plate method, the antimicrobial agents are applied on the surface of solidified, inoculated agar medium, by using filter paper disc and cylinder respectively.
On incubation at the temperature of 30−35°C for 2− 3 days, a zone of inhibition is observed. The diameter of this zone gives an indication of the relative activities of different antimicrobial substances against the test microbes.
Ditch-Plate or Giant Colony Method
A ditch is prepared in agar plate and the solution of antimicrobial substances is carefully poured into the ditch. On the agar surface, a loopful of each test microorganism is streaked outwards from the ditch. Microbes resistant to the antimicrobial agent grow on right of the ditch; whereas susceptible microorganisms show a zone of inhibition adjacent to the ditch or centre of plate.
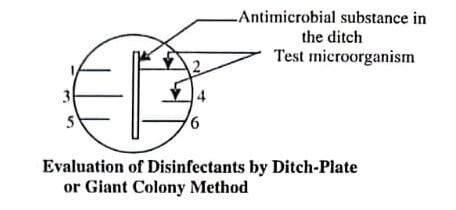
The width of the zone of inhibition (figure) indicates the relative activity of antimicrobial substances against various microbes used during test.
Kelsey-Sykes (KS) Test
This test is the measurement of the capacity of a disinfectant to retain its activity, when it is used repeatedly in different microbiological operations. This test is also known as capacity test.
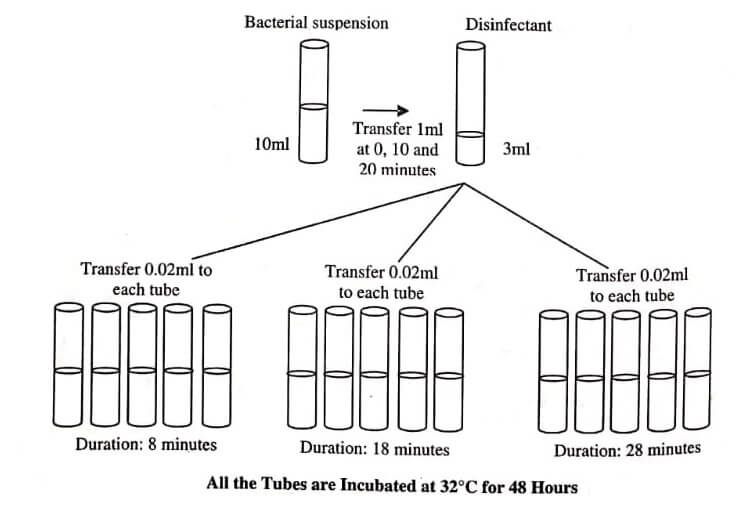
In this test, standard microbes (Esch. coli, Ps. aeruginosa, and Staphylococcus aureus) are added to the disinfectant in three continuous lots at time difference of 0,10 , and 20 minutes. These three lots are kept in contact with disinfectant for 8 minutes and samples are transferred at the difference of 8,18 , and 28 minutes respectively to a recovery medium.
Thus, efficiency of disinfectant is determined by its ability to kill bacteria, and not by comparing with phenol. This test is performed both in dirty and clean conditions; therefore it also measures the effectiveness of a disinfectant in presence of an organic matter.
The selection of the test organism (S.aureus, P. aeruginosa, P. vulgaris, and E. coli) depends on the type of disinfectant used for testing procedure. For carrying the test under ‘clean’ conditions, the dilutions of the disinfectant are made in hard water; whereas, to run the test under ‘dirty’ conditions yeast suspension is used for making dilutions of the disinfectants.
Test organism with yeast or alone is added at time intervals of 0,10 , and 20 minutes. The test organism and the disinfectant are kept in contact for 8 minutes. Three sets of five replicate cultures are made, corresponding to each condition and are incubated at 32°C for 48 hours and growth is assessed by turbidity. Evaluation of the disinfectant is performed on the basis of ability of disinfectant to kill microbes or lack of it; results obtained after this are regarded as a fail or pass.
Results will be negative, if two or more negative cultures are obtained in a single set. In case of negative results, the disinfectant passes the dilution test, after the first and second conditions. The third condition is not considered in fail/pass criterion, but positive cultures serve as inbuilt controls. A lower concentration of the disinfectant may be tested, if negative results are obtained after the third condition.
In-Use Dilution Test
This test is used for examining the quantity of viable organisms, and also for testing the liquid disinfectants used in hospitals. The efficiency of a disinfectant is determined on the basis of its capacity to kill the known number of a standard strain of pathogenic Staphylococcus, within the given time and surface. Results obtained by this test are practically more useful than the results obtained from Rideal-Walker Test and its modifications.
In this test, disinfectants are used in diluted form and the dilution at which the efficiency and potency of the disinfecting agent is determined. The current standards for in-use test are provided by the American Official Analytical Chemists (AOAC) that mentioned the use of three bacterial strains:
- Staphylococcus aureus,
- Salmonella choleraesuis, and
- Pseudomonas aeruginosa.
Standardised cultures of the test organisms are grown in liquid media. This media is standardised and metal carrier rings are dipped into it and removed, and dried at 37°C for a short time. After that, the dried cultures are placed into a disinfectant solution, made at a concentration specified by the manufactures. The culture is left for 10 minutes at 20°C. In the next step, the carrier rings are transferred to a culture medium that allows the growth of any surviving organisms. Number of organisms in the resulting culture is calculated showing the effectiveness of the disinfectants.
Phenol Coefficient Test – Rideal-Walker (RW) Test
In phenol coefficient or Rideal-Walker test, a suspension with similar quantities of organisms is used in the estimation of efficiency and action of different concentration of phenol and the disinfectant to be tested. A solution of the test disinfectant that sterilises the suspension in a given time is divided by the corresponding dilutions of phenol; this gives the phenol coefficient.
However, phenol coefficient is not the measurement of the practical functioning of the test disinfectant in the presence of organic matters.
By Rideal-Walker test (that uses Rideal-Walker broth and Salmonella typhi), the phenol coefficient of test disinfectant may be calculated.
By using the testing disinfectant and phenol, different dilutions are prepared and 5ml of each dilution are inoculated with 0.5ml of the 24 hours broth culture of the organisms.
All incubated tubes (disinfectant + organisms and phenol + organisms) are kept at the temperature of 17.5°C in a water bath. Sub-cultures of each reaction mixture is prepared and transferred to 5ml sterile broth, at time intervals of 2.5,5,7.5, and 10 minutes.
The broth tubes are incubated at the temperature of 37°C for 48−72 hours, and examined for the absence or presence of the growth of microorganisms. A typical example of test result is shown below in table. The Rideal-Walker coefficient of the test disinfectant is calculated. according to the data obtained (shown in table).
| Disinfectant | Dilution | Time Interval for Sub-Culture (Minute) | |||
| 2.5 | 5 | 7.5 | 10 | ||
| Test disinfectant | 1: 1000 | + | – | – | – |
| 1: 2000 | + | + | – | – | |
| 1: 3000 | + | + | + | – | |
| 1: 4000 | + | + | + | + | |
| Phenol | 1 : 80 | + | – | – | – |
| 1 : 100 | + | + | – | – | |
| 1 : 120 | + | + | + | – | |
| 1 : 140 | + | + | + | + | |
R.W. coeffieient ![]()
= 2000/100 = 20
Conclusion
- If the Rideal-Walker or phenol coefficient for a given test disinfectant is 1, the disinfectant has the same effectiveness as phenol.
-
If phenol coefficient of test disinfectant is 20 , the disinfectant is 20 times more active than phenol.
-
If phenol coefficient of test disinfectant is less than 1 , it is less effective; and if more than 1 , it is more effective as compared to phenol.
Chick-Martin Test
The phenol coefficient test is performed on laboratory, level to study the bactericidal action of compound with respect to phenol. In 1908, Chick and Martin suggested that the disinfectants are essential to act in the presence of organic matter, so they recommended the use of dried human faeces in the test system. As a substitute to human faeces, Garrod suggested to use dried yeast.
In this test, Salmonella typhi (test organism) is inoculated into solutions having graded concentrations of test substance (or phenol in case of standard) along with dried yeast. The components are left for 30 minutes at 20°C to interact and lastly duplicate subcultures are made into nutrient broth.
These sub-culture tubes are incubated for 48 hours at 37°C. Observations are made regarding the presence or absence of growth of microorganisms. The phenol concentration that prevents growth in both systems is determined and the mean value is calculated. The same value would be obtained for the unknown. For calculating the coefficient, the value obtained for phenol is divided by the value found for the unknown.
| Read More Topics |
| Embryonated hen’s egg introduction |
| Evaluation of efficiency of sterilization methods |
| Factors influencing disinfection |
| Quantitative measurement of bacterial growth |





